https://pubchem.ncbi.nlm.nih.gov/compound/5988
Sucrose
pubchem.ncbi.nlm.nih.gov
Sucrose 우리가 어릴 때 엄청나게 좋아 했던 사카리에 대한 화학적 이해
PubChem CID
5988
Structure
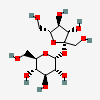


Chemical Safety
Molecular Formula
Synonyms
- sucrose
- 57-50-1
- saccharose
- sugar
- Table sugar
Molecular Weight
342.30 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Dates
-
Create:2004-09-16
-
Modify:2023-11-18
Description
Sucrose appears as white odorless crystalline or powdery solid. Denser than water.
CAMEO Chemicals
Sucrose is a glycosyl glycoside formed by glucose and fructose units joined by an acetal oxygen bridge from hemiacetal of glucose to the hemiketal of the fructose. It has a role as an osmolyte, a sweetening agent, a human metabolite, an algal metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite.
ChEBI
DrugBank
View More...See also: Anise; ferrous disulfide; sucrose (component of); Phosphoric acid; sucrose (component of); Sucrose caramel (related) ... View More ...
1 Structures
1.1 2D Structure
Structure Search
Get Image
Download Coordinates
Chemical Structure Depiction
PubChem
1.2 3D Conformer
Structure Search
Get Image
Download Coordinates
Interactive Chemical Structure Model
Ball and Stick
Sticks
Wire-Frame
Space-Filling
Show Hydrogens
Animate
Full screenZoom inZoom out
FirstPrevious
Conformerof 7
NextLastPubChem
1.3 Crystal Structures
1 of 32
View AllThe Cambridge Structural Database
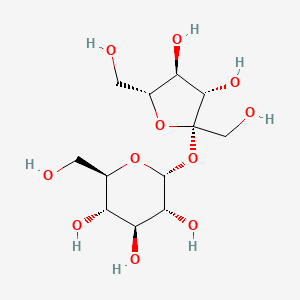
2 Biologic Description
1 of 2
SVG Image
IUPAC Condensed
Glc(a1-2b)Fruf
LINUCS
[][a-D-Glcp]{}
IUPAC
beta-D-arabino-hex-2-ulofuranosyl alpha-D-gluco-hexopyranoside
WURCS
WURCS=2.0/2,2,1/[a2122h-1a_1-5][ha122h-2b_2-5]/1-2/a1-b2
PubChem
2 of 2
GlyCosmos Species
GlyCosmos Monoisotopic Mass
342.12
GlyCosmos Subsumption
GlyCosmos Glycoscience Portal
3 Names and Identifiers
3.1 Computed Descriptors
3.1.1 IUPAC Name
(2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol
Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07)
PubChem
3.1.2 InChI
InChI=1S/C12H22O11/c13-1-4-6(16)8(18)9(19)11(21-4)23-12(3-15)10(20)7(17)5(2-14)22-12/h4-11,13-20H,1-3H2/t4-,5-,6-,7-,8+,9-,10+,11-,12+/m1/s1
Computed by InChI 1.0.6 (PubChem release 2021.05.07)
PubChem
3.1.3 InChIKey
CZMRCDWAGMRECN-UGDNZRGBSA-N
Computed by InChI 1.0.6 (PubChem release 2021.05.07)
PubChem
3.1.4 Canonical SMILES
C(C1C(C(C(C(O1)OC2(C(C(C(O2)CO)O)O)CO)O)O)O)O
Computed by OEChem 2.3.0 (PubChem release 2021.05.07)
PubChem
3.1.5 Isomeric SMILES
C([C@@H]1[C@H]([C@@H]([C@H]([C@H](O1)O[C@]2([C@H]([C@@H]([C@H](O2)CO)O)O)CO)O)O)O)O
Computed by OEChem 2.3.0 (PubChem release 2021.05.07)
PubChem
3.2 Molecular Formula
C12H22O11
Computed by PubChem 2.1 (PubChem release 2021.05.07)
CAMEO Chemicals; PubChem
C12H22O11
ILO-WHO International Chemical Safety Cards (ICSCs)
3.3 Other Identifiers
3.3.1 CAS
57-50-1
CAMEO Chemicals; CAS Common Chemistry; ChemIDplus; DrugBank; EPA Chemicals under the TSCA; EPA DSSTox; European Chemicals Agency (ECHA); FDA Global Substance Registration System (GSRS); Hazardous Substances Data Bank (HSDB); Human Metabolome Database (HMDB); ILO-WHO International Chemical Safety Cards (ICSCs); Occupational Safety and Health Administration (OSHA); The National Institute for Occupational Safety and Health (NIOSH)
3.3.2 Related CAS
3.3.3 Deprecated CAS
100405-08-1, 104242-10-6, 131932-12-2, 146054-35-5, 146187-04-4, 151756-02-4, 220376-22-7, 29253-78-9, 29764-06-5, 30027-72-6, 47167-52-2, 47185-09-1, 47257-91-0, 50857-68-6, 51909-69-4, 64533-66-0, 65545-99-5, 75398-84-4, 76056-38-7, 78654-77-0, 80165-03-3, 8027-47-2, 8030-20-4, 85456-51-5, 86101-30-6, 87430-66-8, 92004-84-7, 635681-90-2, 786702-63-4, 12040-73-2, 1159795-78-4, 1206156-82-2, 880257-62-5, 1192061-43-0, 1481578-88-4, 1566560-95-9, 2169316-40-7
ChemIDplus
146054-35-5, 78654-77-0, 100405-08-1, 30027-72-6, 75398-84-4, 51909-69-4, 146187-04-4, 29764-06-5, 47257-91-0, 104242-10-6, 50857-68-6, 47167-52-2, 86101-30-6, 1159795-78-4, 87430-66-8, 131932-12-2, 8027-47-2, 12040-73-2, 1206156-82-2, 29253-78-9, 64533-66-0, 65545-99-5, 8030-20-4, 76056-38-7, 80165-03-3, 85456-51-5, 786702-63-4, 880257-62-5, 220376-22-7, 151756-02-4, 635681-90-2, 92004-84-7, 47185-09-1
EPA DSSTox
3.3.4 European Community (EC) Number
European Chemicals Agency (ECHA)
3.3.5 ICSC Number
ILO-WHO International Chemical Safety Cards (ICSCs)
3.3.6 RTECS Number
The National Institute for Occupational Safety and Health (NIOSH)
3.3.7 UNII
FDA Global Substance Registration System (GSRS)
3.3.8 DSSTox Substance ID
EPA DSSTox
3.3.9 Nikkaji Number
Japan Chemical Substance Dictionary (Nikkaji)
3.3.10 Wikipedia
Wikipedia
3.3.11 Wikidata
Wikidata
3.3.12 GlyTouCan Accession
G05551OP
GlyCosmos Glycoscience Portal; GlyTouCan Project
G78152OD
GlyTouCan Project
G44791DH
GlyTouCan Project
3.3.13 NCI Thesaurus Code
NCI Thesaurus (NCIt)
3.3.14 RXCUI
NLM RxNorm Terminology
3.3.15 Metabolomics Workbench ID
Metabolomics Workbench
3.3.16 ChEMBL ID
ChEMBL
3.3.17 KEGG ID
3.4 Synonyms
3.4.1 MeSH Entry Terms
- Saccharose
- Sucrose
Medical Subject Headings (MeSH)
3.4.2 Depositor-Supplied Synonyms
- sucrose
- 57-50-1
- saccharose
- sugar
- Table sugar
- Cane sugar
- White sugar
- D-Sucrose
- Rohrzucker
- Saccharum
- Microse
- Rock candy
- Amerfand
- Amerfond
- Confectioner's sugar
- D-(+)-Saccharose
- Sucrose, pure
- sacarosa
- D(+)-Sucrose
- Sucrose, dust
- D(+)-Saccharose
- Sacharose
- D-(+)-Sucrose
- beta-D-Fructofuranosyl-alpha-D-glucopyranoside
- D-Saccharose
- CCRIS 2120
- HSDB 500
- Sucraloxum [INN-Latin]
- CHEBI:17992
- beta-D-Fructofuranosyl alpha-D-glucopyranoside
- NCI-C56597
- (+)-Sucrose
- AI3-09085
- alpha-D-Glucopyranosyl beta-D-fructofuranoside
- Sucrose, purified
- (alpha-D-Glucosido)-beta-D-fructofuranoside
- EINECS 200-334-9
- NSC 406942
- Fructofuranoside, alpha-D-glucopyranosyl, beta-D
- Glucopyranoside, beta-D-fructofuranosyl, alpha-D
- DTXSID2021288
- UNII-C151H8M554
- GNE-410
- S-67F
- Glc(alpha1->2beta)Fru
- alpha-D-Glucopyranoside, beta-D-fructofuranosyl-
- C151H8M554
- NSC-406942
- DTXCID101288
- 1-alpha-D-glucopyranosyl-2-beta-D-fructofuranoside
- alpha-D-Glucopyranoside, beta-D-fructofuranosyl
- beta-D-Fruf-(2<->1)-alpha-D-Glcp
- NCGC00164248-01
- Sucraloxum
- Sucraloxum (INN-Latin)
- SUCROSE (II)
- SUCROSE [II]
- SUCROSE (USP-RS)
- SUCROSE [USP-RS]
- SUCROSE (EP IMPURITY)
- SUCROSE [EP IMPURITY]
- SUCROSE (EP MONOGRAPH)
- SUCROSE [EP MONOGRAPH]
- Saccarose
- Sucrose [USAN:JAN]
- MFCD00006626
- CAS-57-50-1
- (2R,3R,4S,5S,6R)-2-{[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-6-(hydroxymethyl)oxane-3,4,5-triol
- Sucrose [JAN:NF]
- Beetsugar
- GLC-(1-2)FRU
- Frost Sugar
- Sucrose,ultrapure
- Manalox AS
- Compressible sugar
- Sucrose, AR
- Sucrose, LR
- Sucrose, ultrapure
- Sucrose, USP
- Sucrose ACS grade
- Sucrose (TN)
- Sugar spheres (NF)
- Sugar,(S)
- REFINED SUGAR
- Sucrose, ACS reagent
- Sucrose, reagent grade
- 1af6
- SUGAR, WHITE
- SUCROSE [VANDF]
- Sucrose (for injection)
- SUCROSE [HSDB]
- SUCROSE [INCI]
- DYSPEPSIA HEADACHE
- Sucrose (JP17/NF)
- SUCROSE [FCC]
- SUCROSE [JAN]
- SUGAR [VANDF]
- SUCROSE [MI]
- SUCROSE [NF]
- Sucrose Biochemical grade
- SUCROSE [WHO-DD]
- Sucrose, SAJ first grade
- SACCHARUM OFFICINALE
- Sugar, compressible (NF)
- bmse000119
- bmse000804
- bmse000918
- Epitope ID:153236
- Sucrose, >=99.5%
- Sucrose, JIS special grade
- White soft sugar (JP17)
- Sucrose, analytical standard
- Sucrose, cell culture tested
- Sugar, confectioner's (NF)
- 1-alpha-D-glucopyranosyl-2-beta-D-fructofranoside
- Sucrose, p.a., ACS reagent
- CHEMBL253582
- GTPL5411
- CHEBI:65313
- Sucrose, Molecular Biology Grade
- CZMRCDWAGMRECN-UGDNZRGBSA-N
- Sucrose, >=99.5% (GC)
- alpha-D-Glc-(1-2)-beta-D-Fru
- SACCHARUM OFFICINALE [HPUS]
- HY-B1779
- Tox21_112093
- Tox21_201397
- Tox21_300410
- BDBM50108105
- s3598
- Sucrose, for electrophoresis, >99%
- AKOS024306988
- DB02772
- Sucrose, BioXtra, >=99.5% (GC)
- a-D-Glucopyranosyl A-D-fructofuranoside
- b -D-Fructofuranosyl a-D-glucopyranoside
- NCGC00164248-02
- NCGC00164248-03
- NCGC00164248-05
- NCGC00254237-01
- NCGC00258948-01
- (2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol
- D-Saccharose 20000 microg/mL in Water
- Sucrose, meets USP testing specifications
- Sucrose, Vetec(TM) reagent grade, 99%
- D-Saccharose 1000 microg/mL in Methanol
- alpha-D-Glucopyranosylbeta-D-fructofuranoside
- CS-0013810
- S0111
- Sucrose, Grade I, plant cell culture tested
- Sucrose, Grade II, plant cell culture tested
- C00089
- D00025
- D70407
- EN300-126630
- Sucrose, for molecular biology, >=99.5% (GC)
- Sucrose|?-D-Fructofuranosyl ?-D-glucopyranoside
- SR-01000883983
- Sucrose, NIST(R) SRM(R) 17f, optical rotation
- J-519846
- Q4027534
- SR-01000883983-1
- Sucrose, for microbiology, ACS reagent, >=99.0%
- alpha-D-glucopyranosyl-(1->2)-beta-D-fructofuranoside
- Sucrose, British Pharmacopoeia (BP) Reference Standard
- Sucrose, European Pharmacopoeia (EP) Reference Standard
- Sucrose, Vetec(TM) reagent grade, RNase and DNase free
- Z1589255958
- .BETA.-D-FRUCTOFURANOSYL-.ALPHA.-D-GLUCOPYRANOSIDE
- beta-D-fructofuranosyl-(2↔1)-alpha-D-glucopyranoside
- Sucrose, analytical standard, for enzymatic assay kit SCA20
- .ALPHA.-D-GLUCOPYRANOSIDE, .BETA.-D-FRUCTOFURANOSYL-
- SUCROSE (CONSTITUENT OF CRANBERRY LIQUID PREPARATION)
- Sucrose, anhydrous, free-flowing, Redi-Dri(TM), ACS reagent
- Sucrose, BioUltra, for molecular biology, >=99.5% (HPLC)
- Sucrose, United States Pharmacopeia (USP) Reference Standard
- Carbon isotopes in sucrose, NIST(R) RM 8542, IAEA-CH-6 sucrose
- SUCROSE (CONSTITUENT OF CRANBERRY LIQUID PREPARATION) [DSC]
- Compressible sugar, United States Pharmacopeia (USP) Reference Standard
- Sucrose, puriss., meets analytical specification of Ph. Eur., BP, NF
- WURCS=2.0/2,2,1/[ha122h-2b_2-5][a2122h-1a_1-5]/1-2/a2-b1
- (2R,3R,4S,5S,6R)-2-(((2S,3S,4S,5R)-3,4-Dihydroxy-2,5-bis(hydroxymethyl)tetrahydrofuran-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol
- (2R,3R,4S,5S,6R)-2-((2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)tetrahydrofuran-2-ylhydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol
- (2R,3R,4S,5S,6R)-2-((2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)tetrahydrofuran-2-yloxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol
- (2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)tetrahydrofuran-2-yl]oxy-6-(hydroxymethyl)tetrahydropyran-3,4,5-triol
- 8027-47-2
- 8030-20-4
- 85456-51-5
- 86101-30-6
- 87430-66-8
- 92004-84-7
- A-5
- Sucrose, BioReagent, suitable for cell culture, suitable for insect cell culture, >=99.5% (GC)
PubChem
4 Chemical and Physical Properties
4.1 Computed Properties
Property Name
Property Value
Reference
Molecular Weight
342.30 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
XLogP3
-3.7
Computed by XLogP3 3.0 (PubChem release 2021.05.07)
Hydrogen Bond Donor Count
8
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Hydrogen Bond Acceptor Count
11
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Rotatable Bond Count
5
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Exact Mass
342.11621151 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Monoisotopic Mass
342.11621151 g/mol
Computed by PubChem 2.1 (PubChem release 2021.05.07)
Topological Polar Surface Area
190Ų
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Heavy Atom Count
23
Computed by PubChem
Formal Charge
0
Computed by PubChem
Complexity
395
Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)
Isotope Atom Count
0
Computed by PubChem
Defined Atom Stereocenter Count
9
Computed by PubChem
Undefined Atom Stereocenter Count
0
Computed by PubChem
Defined Bond Stereocenter Count
0
Computed by PubChem
Undefined Bond Stereocenter Count
0
Computed by PubChem
Covalently-Bonded Unit Count
1
Computed by PubChem
Compound Is Canonicalized
Yes
Computed by PubChem (release 2021.05.07)
PubChem
4.2 Experimental Properties
4.2.1 Physical Description
Sucrose appears as white odorless crystalline or powdery solid. Denser than water.
CAMEO Chemicals
EPA Chemicals under the TSCA
Hard, white, odorless crystals, lumps, or powder. [Note: May have a characteristic, caramel odor when heated.]; [NIOSH]
Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
Solid
Human Metabolome Database (HMDB)
WHITE SOLID IN VARIOUS FORMS.
ILO-WHO International Chemical Safety Cards (ICSCs)
Hard, white, odorless crystals, lumps, or powder.
Occupational Safety and Health Administration (OSHA)
Hard, white, odorless crystals, lumps, or powder. [Note: May have a characteristic, caramel odor when heated.]
The National Institute for Occupational Safety and Health (NIOSH)
4.2.2 Color / Form
Monoclinic sphenoidal crystals, crystalline masses, blocks, or powder
Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 1517
Hazardous Substances Data Bank (HSDB)
Hard, white ... crystals, lumps, or powder ...
NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 288
Hazardous Substances Data Bank (HSDB)
4.2.3 Odor
Characteristic caramel
Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 1518
Hazardous Substances Data Bank (HSDB)
... Odorless ... [Note: May have a characteristic caramel odor when heated].
NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 288
Hazardous Substances Data Bank (HSDB)
4.2.4 Taste
Sweet
Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 1517
Hazardous Substances Data Bank (HSDB)
4.2.5 Boiling Point
Decomposes (NTP, 1992)
National Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health (NTP). 1992. National Toxicology Program Chemical Repository Database. Research Triangle Park, North Carolina.
CAMEO Chemicals
decomposes
Occupational Safety and Health Administration (OSHA); The National Institute for Occupational Safety and Health (NIOSH)
4.2.6 Melting Point
320 to 367 °F (decomposes) (NTP, 1992)
National Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health (NTP). 1992. National Toxicology Program Chemical Repository Database. Research Triangle Park, North Carolina.
CAMEO Chemicals
185.5°C
PhysProp
DrugBank
185.5 °C
Lide, D.R. (ed.). CRC Handbook of Chemistry and Physics. 79th ed. Boca Raton, FL: CRC Press Inc., 1998-1999., p. 3-172
Hazardous Substances Data Bank (HSDB); Human Metabolome Database (HMDB)
320-367 °F (decomposes)
Occupational Safety and Health Administration (OSHA); The National Institute for Occupational Safety and Health (NIOSH)
4.2.7 Solubility
greater than or equal to 100 mg/mL at 66 °F (NTP, 1992)
National Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health (NTP). 1992. National Toxicology Program Chemical Repository Database. Research Triangle Park, North Carolina.
CAMEO Chemicals
2100000mg/L (at 25 °C)
YALKOWSKY,SH & DANNENFELSER,RM (1992)
DrugBank
Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 1518
Hazardous Substances Data Bank (HSDB)
Lide, D.R. (ed.). CRC Handbook of Chemistry and Physics. 79th ed. Boca Raton, FL: CRC Press Inc., 1998-1999., p. 3-172
Hazardous Substances Data Bank (HSDB)
water solubility = 2.12X10+6 mg/l @ 25 °C
Yalkowsky SH, Dannenfelser RM; The AQUASOL dATAbASE of Aqueous Solubility. Fifth Ed, Tucson, AZ: Univ Az, College of Pharmacy (1992)
Hazardous Substances Data Bank (HSDB)
2100.0 mg/mL
Human Metabolome Database (HMDB)
Solubility in water, g/100ml at 25 °C: 200
ILO-WHO International Chemical Safety Cards (ICSCs)
200%
The National Institute for Occupational Safety and Health (NIOSH)
4.2.8 Density
1.59 at 68 °F (USCG, 1999) - Denser than water; will sink
U.S. Coast Guard. 1999. Chemical Hazard Response Information System (CHRIS) - Hazardous Chemical Data. Commandant Instruction 16465.12C. Washington, D.C.: U.S. Government Printing Office.
CAMEO Chemicals
1.5805 g/cu cm @ 17 °C
Lide, D.R. (ed.). CRC Handbook of Chemistry and Physics. 79th ed. Boca Raton, FL: CRC Press Inc., 1998-1999., p. 3-172
Hazardous Substances Data Bank (HSDB)
1.6 g/cm³
ILO-WHO International Chemical Safety Cards (ICSCs)
1.59
Occupational Safety and Health Administration (OSHA); The National Institute for Occupational Safety and Health (NIOSH)
4.2.9 Vapor Pressure
0 mmHg (approx) (NIOSH, 2023)
CAMEO Chemicals
0 mmHg (approx)
Occupational Safety and Health Administration (OSHA); The National Institute for Occupational Safety and Health (NIOSH)
4.2.10 LogP
-3.7
HANSCH,C ET AL. (1995)
DrugBank
log Kow = -3.70
Hansch, C., Leo, A., D. Hoekman. Exploring QSAR - Hydrophobic, Electronic, and Steric Constants. Washington, DC: American Chemical Society., 1995., p. 107
Hazardous Substances Data Bank (HSDB)
-3.70
HANSCH,C ET AL. (1995)
Human Metabolome Database (HMDB)
-3.67
ILO-WHO International Chemical Safety Cards (ICSCs)
4.2.11 Stability / Shelf Life
STABLE IN AIR
The Merck Index. 9th ed. Rahway, New Jersey: Merck & Co., Inc., 1976., p. 1149
Hazardous Substances Data Bank (HSDB)
4.2.12 Decomposition
When heated to decomposition it emits acrid smoke and fumes.
Sax, N.I. Dangerous Properties of Industrial Materials. 6th ed. New York, NY: Van Nostrand Reinhold, 1984., p. 2478
Hazardous Substances Data Bank (HSDB)
186 °C
ILO-WHO International Chemical Safety Cards (ICSCs)
4.2.13 Heat of Combustion
-1.35X10+6 cal/mol
Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V21 867
Hazardous Substances Data Bank (HSDB)
4.2.14 pH
Soln are neutral to litmus
Lewis, R.J., Sr (Ed.). Hawley's Condensed Chemical Dictionary. 13th ed. New York, NY: John Wiley & Sons, Inc. 1997., p. 1057
Hazardous Substances Data Bank (HSDB)
4.2.15 Surface Tension
71-75 mN/m @ 1-0.6 mol/l
Gerhartz, W. (exec ed.). Ullmann's Encyclopedia of Industrial Chemistry. 5th ed.Vol A1: Deerfield Beach, FL: VCH Publishers, 1985 to Present., p. VB1 (90) 6-54
Hazardous Substances Data Bank (HSDB)
4.2.16 Refractive Index
INDEX OF REFRACTION: 1.5376; SADTLER REFERENCE NUMBER: 8659 (IR, PRISM), 563 (IR, GRATING); SPECIFIC OPTICAL ROTATION: +66.37 @ 20 °C/D (WATER)
Weast, R.C. (ed.). Handbook of Chemistry and Physics. 60th ed. Boca Raton, Florida: CRC Press Inc., 1979., p. C-503
Hazardous Substances Data Bank (HSDB)
4.2.17 Caco2 Permeability
-5.77
ADME Research, USCD
DrugBank
'화학(공학)' 카테고리의 다른 글
| 휘발성 유기 화합물 (1) | 2023.11.24 |
|---|---|
| aspirin; ACETYLSALICYLIC ACID (0) | 2023.11.23 |
| Biochemicals (1) | 2023.11.23 |
| 유럽 폴리머 저널 (1) | 2023.06.02 |
| 화학 (0) | 2023.04.04 |




