Biotechnology 생명공학

Biotechnology is a broad area of biology, involving the use of living systems and organisms to develop or make products. 생명공학은 생물학의 넓은 영역으로, 생물체들과 생물체들이 제품을 개발하거나 만들기 위해 사용하는 것을 포함한다. Depending on the tools and applications, it often overlaps with related scientific fields. 도구와 용도에 따라 관련 과학 분야와 중복되는 경우가 많다. In the late 20th and early 21st centuries, biotechnology has expanded to include new and diverse sciences, such as genomics, recombinant gene techniques, applied immunology, and development of pharmaceutical therapies and diagnostic tests. 20세기 후반과 21세기 초반에 생명공학은 유전체학, 재조합 유전자 기법, 응용면역학, 제약요법과 진단시험의 개발 등 새롭고 다양한 과학으로 확대되었다. The term biotechnology was first used by Karl Ereky in 1919, meaning the production of products from raw materials with the aid of living organisms. 생명공학이라는 용어는 1919년 칼 에레키에 의해 처음 사용되었는데, 살아있는 유기체의 도움을 받아 원료에서 생산되는 제품을 생산한다는 뜻이다.
Contents 내용물
- 1 Definition정의
- 2 History역사
- 3 Examples예
- 4 Learning배우는 것은
- 5 References and notes참조 및 참고 사항
- 6 External links외부 링크
Definition정의[[edit편집]]
The wide concept of biotechnology encompasses a wide range of procedures for modifying living organisms according to human purposes, going back to domestication of animals, cultivation of the plants, and "improvements" to these through breeding programs that employ artificial selection and hybridization.생명공학의 넓은 개념은 인간의 목적에 따라 생물체를 개조하는 광범위한 절차, 동물의 가축화, 식물의 재배, 인공 선택과 잡종을 채용하는 사육 프로그램을 통해 이를 '개선'하는 과정을 포괄한다. Modern usage also includes genetic engineering as well as cell and tissue culture technologies. 현대 용법에는 세포와 조직 배양 기술뿐만 아니라 유전 공학도 포함된다. The American Chemical Society defines biotechnology as the application of biological organisms, systems, or processes by various industries to learning about the science of life and the improvement of the value of materials and organisms such as pharmaceuticals, crops, and livestock.[1] 미국화학회는 생명공학이 생명과학과 제약, 농작물, 가축 등 물질과 생물의 가치향상을 위해 다양한 산업에 의한 생물학적 유기체, 시스템 또는 공정을 응용한 것으로 정의하고 있다.[1] Per the European Federation of Biotechnology, biotechnology is the integration of natural science and organisms, cells, parts thereof, and molecular analogues for products and services.[2] 유럽 생명공학 연맹에 따르면, 생명공학은 자연과학과 유기체, 세포, 세포의 일부, 그리고 제품과 서비스에 대한 분자 유사성의 통합이다.[2] Biotechnology is based on the basic biological sciences (e.g. molecular biology, biochemistry, cell biology, embryology, genetics, microbiology) and conversely provides methods to support and perform basic research in biology. 생명공학은 기초생물학(예: 분자생물학, 생화학, 세포생물학, 발생학, 유전학, 미생물학)을 기반으로 하며, 반대로 생물학의 기초연구를 지원하고 수행할 수 있는 방법을 제공한다.
Biotechnology is the research and development in the laboratory using bioinformatics for exploration, extraction, exploitation and production from any living organisms and any source of biomass by means of biochemical engineering where high value-added products could be planned (reproduced by biosynthesis, for example), forecasted, formulated, deve생명공학은 고부가가치 제품을 계획할 수 있는 생화학 공학을 이용하여(예를 들어, 생물합성술에 의해 재생산됨), 예측, 공식화, 디브이(deve)하는 모든 생물체 및 바이오매스의 출처로부터 탐사, 추출, 착취 및 생산을 위해 생물정보학을 사용하는 실험실의 연구 개발이다.loped, manufactured, and marketed for the purpose of sustainable operations (for the return from bottomless initial investment on R & D) and gaining durable patents rights (for exclusives rights for sales, and prior to this to receive national and international approval from the results on animal experiment and human experiment, especially on the p, 제조하고 지속 가능한 작전(R및 깊이를 알 수 없는 초기 투자에서 돌아오는;D에)고라고 했잖아요 독점권 권리 위하는 내구 특허 권리(의 목적으로 내세웠던 Loped.를 판매와 이에 앞서 동물 실험과 인간의 실험의 결과에서 페이지의 주 특히 국가 및 국제적 승인을 받은harmaceutical branch of biotechnology to prevent any undetected side-effects or safety concerns by using the products).[3][4][5]제품을 사용하여 탐지되지 않은 측면 장애물 또는 안전 문제를 방지하기 위한 생명공학의 제약 부문).[3][4][5] The utilization of biological processes, organisms or systems to produce products that are anticipated to improve human lives is termed biotechnology.[6] 생물학적 과정, 유기체 또는 시스템을 이용하여 인간의 삶을 개선할 것으로 기대되는 제품을 생산하는 것을 생명공학이라고 한다.[6]
By contrast, bioengineering is generally thought of as a related field that more heavily emphasizes higher systems approaches (not necessarily the altering or using of biological materials directly) for interfacing with and utilizing living things.이와는 대조적으로, 생명공학은 일반적으로 생물과의 인터페이스와 활용을 위해 더 높은 시스템 접근법(생물학적 물질의 직접 변경 또는 사용)을 더 많이 강조하는 관련 분야로 생각된다. Bioengineering is the application of the principles of engineering and natural sciences to tissues, cells and molecules. 생명공학은 공학과 자연과학의 원리를 조직, 세포, 분자에 적용하는 것이다. This can be considered as the use of knowledge from working with and manipulating biology to achieve a result that can improve functions in plants and animals.[7] 이것은 식물과 동물의 기능을 향상시킬 수 있는 결과를 얻기 위해 생물학을 연구하고 조작함으로써 얻은 지식의 이용이라고 볼 수 있다.[7] Relatedly, biomedical engineering is an overlapping field that often draws upon and applies biotechnology (by various definitions), especially in certain sub-fields of biomedical or chemical engineering such as tissue engineering, biopharmaceutical engineering, and genetic engineering. 이와 관련, 바이오의약품공학은 특히 조직공학, 바이오의약품공학, 유전공학 등 바이오의약품이나 화학공학의 특정 하위분야에서 생명공학(다양한 정의에 의한)을 자주 끌어다가 적용하는 중복분야다.
History역사[[edit편집]]

Brewing was an early application of biotechnology.양조는 생명공학의 초기 응용이었다.
Main article:주요 기사: History of biotechnology 생명공학의 역사
Although not normally what first comes to mind, many forms of human-derived agriculture clearly fit the broad definition of "'utilizing a biotechnological system to make products".비록 일반적으로 가장 먼저 떠오르는 것은 아니지만, 많은 형태의 인간 유래 농업은 '생체공학 시스템을 활용하여 제품을 만든다'라는 넓은 정의에 부합한다. Indeed, the cultivation of plants may be viewed as the earliest biotechnological enterprise. 사실, 식물의 재배는 가장 초기 생명공학적인 사업으로 여겨질 수 있다.
Agriculture has been theorized to have become the dominant way of producing food since the Neolithic Revolution.농업은 신석기 혁명 이후 식량을 생산하는 지배적인 방법이 되었다고 이론화되었다. Through early biotechnology, the earliest farmers selected and bred the best suited crops, having the highest yields, to produce enough food to support a growing population. 초기 생명공학을 통해, 초기 농부들은 증가하는 인구를 부양할 충분한 식량을 생산하기 위해 가장 적합한 작물을 선별하고 번식시켰다. As crops and fields became increasingly large and difficult to maintain, it was discovered that specific organisms and their by-products could effectively fertilize, restore nitrogen, and control pests. 농작물과 밭이 점점 커지고 유지하기가 어려워지면서 특정 생물과 그 부산물이 효과적으로 비료와 질소 복원, 해충 방제를 할 수 있다는 사실이 밝혀졌다. Throughout the history of agriculture, farmers have inadvertently altered the genetics of their crops through introducing them to new environments and breeding them with other plants — one of the first forms of biotechnology. 농업의 역사를 통틀어, 농부들은 농작물을 새로운 환경에 도입하고 다른 식물들과 번식시킴으로써 의도치 않게 농작물의 유전학을 변형시켰는데, 이는 최초의 생명공학 형태 중 하나이다.
These processes also were included in early fermentation of beer.[8]이러한 과정은 맥주의 조기 발효에도 포함되었다.[8] These processes were introduced in early Mesopotamia, Egypt, China and India, and still use the same basic biological methods. 이러한 과정들은 초기 메소포타미아, 이집트, 중국, 인도에서 도입되었으며, 여전히 같은 기본적인 생물학적 방법을 사용하고 있다. In brewing, malted grains (containing enzymes) convert starch from grains into sugar and then adding specific yeasts to produce beer. 양조할 때, 맥아 알갱이(포함효소)는 곡물의 전분을 설탕으로 변환한 다음 특정한 효모를 첨가하여 맥주를 생산한다. In this process, carbohydrates in the grains broke down into alcohols,e such as ethanol. 이 과정에서, 곡물 속의 탄수화물은 에탄올과 같은 알코올로 분해되었다. Later, other cultures produced the process of lactic acid fermentation, which produced other preserved foods, such as soy sauce. 후에 다른 문화권에서는 젖산 발효 과정을 만들어 냈는데, 이 과정에서 간장과 같은 다른 보존식품이 생산되었다. Fermentation was also used in this time period to produce leavened bread. 발효는 또한 이 시기에 발효된 빵을 생산하는데 사용되었다. Although the process of fermentation was not fully understood until Louis Pasteur's work in 1857, it is still the first use of biotechnology to convert a food source into another form. 비록 발효 과정이 1857년 루이 파스퇴르의 연구 전까지 완전히 이해되지는 않았지만, 식재료를 다른 형태로 전환하는 것은 여전히 생명공학의 첫 번째 사용이다.
Before the time of Charles Darwin's work and life, animal and plant scientists had already used selective breeding.찰스 다윈의 일과 삶이 시작되기 전, 동물과 식물 과학자들은 이미 선택적 번식을 이용했다. Darwin added to that body of work with his scientific observations about the ability of science to change species. 다윈은 종을 변화시키는 과학의 능력에 대한 과학적인 관찰로 그 연구체를 더했다. These accounts contributed to Darwin's theory of natural selection.[9] 이러한 설명들은 다윈의 자연 선택 이론에 기여했다.[9]
For thousands of years, humans have used selective breeding to improve the production of crops and livestock to use them for food.수천 년 동안 인간은 선택적 번식을 통해 농작물과 가축의 생산을 향상시켜 식용으로 사용해 왔다. In selective breeding, organisms with desirable characteristics are mated to produce offspring with the same characteristics. 선택적 사육에서는 바람직한 특성을 가진 유기체를 결합하여 동일한 특성을 가진 자손을 생산한다. For example, this technique was used with corn to produce the largest and sweetest crops.[10] 예를 들어, 이 기술은 옥수수와 함께 가장 크고 달콤한 농작물을 생산하기 위해 사용되었다.[10]
In the early twentieth century scientists gained a greater understanding of microbiology and explored ways of manufacturing specific products.20세기 초에 과학자들은 미생물학에 대한 이해를 높이고 특정 제품을 제조하는 방법을 탐구했다. In 1917, Chaim Weizmann first used a pure microbiological culture in an industrial process, that of manufacturing corn starch using Clostridium acetobutylicum, to produce acetone, which the United Kingdom desperately needed to manufacture explosives during World War I.[11] 1917년 차임 와이즈만은 제1차 세계대전 당시 영국이 폭발물 제조에 절실한 아세톤을 생산하기 위해 클로스트리디움 아세토부틸리쿰을 이용해 옥수수 전분을 제조하는 산업 공정에서 순수한 미생물학적 문화를 처음 사용했다.[11]
Biotechnology has also led to the development of antibiotics.생명공학은 항생제 개발로도 이어졌다. In 1928, Alexander Fleming discovered the mold Penicillium. 1928년에 알렉산더 플레밍은 페니실리움 곰팡이를 발견했다. His work led to the purification of the antibiotic compound formed by the mold by Howard Florey, Ernst Boris Chain and Norman Heatley – to form what we today know as penicillin. 그의 연구는 하워드 플로리, 에른스트 보리스 체인과 노먼 히틀리에 의해 형성된 항생제 화합물을 정화시켜 오늘날 우리가 페니실린으로 알고 있는 것을 형성하게 했다. In 1940, penicillin became available for medicinal use to treat bacterial infections in humans.[10] 1940년에 페니실린은 인간의 박테리아 감염을 치료하기 위해 약용으로 사용할 수 있게 되었다.[10]
The field of modern biotechnology is generally thought of as having been born in 1971 when Paul Berg's (Stanford) experiments in gene splicing had early success.현대 생명공학 분야는 일반적으로 유전자 스플라이싱에 대한 폴 버그(스탠포드)의 실험이 초기 성공을 거두었던 1971년에 태어난 것으로 생각된다. Herbert W. 허버트 W. Boyer (Univ. 보이어(유니브). Calif. at San Francisco) and Stanley N. 캘리포니아 주(샌프란시스코)와 스탠리 N. Cohen (Stanford) significantly advanced the new technology in 1972 by transferring genetic material into a bacterium, such that the imported material would be reproduced. 코헨(스탠포드)은 1972년 수입된 물질이 재생산될 수 있도록 유전 물질을 박테리아로 옮겨 신기술을 대폭 발전시켰다. The commercial viability of a biotechnology industry was significantly expanded on June 16, 1980, when the United States Supreme Court ruled that a genetically modified microorganism could be patented in the case of Diamond v. Chakrabarty.[12] 생명공학 산업의 상업적 생존가능성은 1980년 6월 16일 미국 대법원이 다이아몬드 대 차크라바티 사건에서 유전자 변형 미생물이 특허를 받을 수 있다고 판결하면서 크게 확대되었다.[12] Indian-born Ananda Chakrabarty, working for General Electric, had modified a bacterium (of the genus Pseudomonas) capable of breaking down crude oil, which he proposed to use in treating oil spills. (Chakrabarty's work did not involve gene manipulation but rather the transfer of entire organelles between strains of the Pseudomonas bacterium. 제너럴 일렉트릭(General Electric)에서 일하는 인도 태생 아난다 차크라바티는 원유 분해 능력이 있는 박테리아(Phyomonas)를 변형시켰는데, 그는 이를 기름 유출 치료에 사용하자고 제안했다.(차크라바티의 작업은 유전자 조작이 아니라 오히려 필로모나스 박테리아 균주의 변종들 사이에 전체 오르간세포가 전이되는 것을 포함했다.
The MOSFET (metal-oxide-semiconductor field-effect transistor) was invented by Mohamed M.MOSFET는 모하메드 M에 의해 발명되었다. Atalla and Dawon Kahng in 1959.[13] 1959년 아탈라와 다원카엥.[13] Two years later, Leland C. 2년 후, Leland C. Clark and Champ Lyons invented the first biosensor in 1962.[14][15] 클라크와 챔프 라이온스는 1962년에 최초의 바이오센서를 발명했다.[14][15] Biosensor MOSFETs were later developed, and they have since been widely used to measure physical, chemical, biological and environmental parameters.[16] 바이오센서 MOSFET는 나중에 개발되었고, 그 이후로 물리, 화학, 생물학적, 환경적 변수를 측정하는 데 널리 사용되어 왔다.[16] The first BioFET was the ion-sensitive field-effect transistor (ISFET), invented by Piet Bergveld in 1970.[17][18] 최초의 바이오FET는 1970년 피에트 베르그벨트에 의해 발명된 이온민감 전계효과 트랜지스터(ISFET)이다.[17][18] It is a special type of MOSFET,[16] where the metal gate is replaced by an ion-sensitive membrane, electrolyte solution and reference electrode.[19] 금속 게이트를 이온감응막, 전해액, 기준 전극으로 대체하는 [16]MOSFET의 특수한 유형이다.[19] The ISFET is widely used in biomedical applications, such as the detection of DNA hybridization, biomarker detection from blood, antibody detection, glucose measurement, pH sensing, and genetic technology.[19] ISFET는 DNA 잡종 검출, 혈액에서 바이오마커 검출, 항체 검출, 포도당 측정, pH 감지, 유전자 기술 등 바이오메디컬 용도에 널리 사용된다.[19]
By the mid-1980s, other BioFETs had been developed, including the gas sensor FET (GASFET), pressure sensor FET (PRESSFET), chemical field-effect transistor (ChemFET), reference ISFET (REFET), enzyme-modified FET (ENFET) and immunologically modified FET (IMFET).[16]1980년대 중반까지 가스 센서 FET(가스펫), 압력 센서 FET(PRESFET), 화학 전계 효과 트랜지스터(켐펫), 참조 ISFET(REFET), 효소 변형 FET(ENFET), 면역 변형 FET(IMFET) 등 다른 바이오 FET가 개발되었다.[16] By the early 2000s, BioFETs such as the DNA field-effect transistor (DNAFET), gene-modified FET (GenFET) and cell-potential BioFET (CPFET) had been developed.[19] 2000년대 초반까지 DNA 전계효과 트랜지스터(DNAFET), 유전자변형 FET(GenFET), 세포전위 바이오FET(CPFET) 등 바이오FET가 개발됐다.[19]
A factor influencing the biotechnology sector's success is improved intellectual property rights legislation—and enforcement—worldwide, as well as strengthened demand for medical and pharmaceutical products to cope with an ageing, and ailing, U.S. population.[20]생명공학 분야의 성공에 영향을 미치는 요인은 고령화와 병든 미국 인구에 대처하기 위한 의료 및 의약품에 대한 수요의 강화와 더불어 전 세계적으로 지적 재산권 및 시행 개선이다.[20]
Rising demand for biofuels is expected to be good news for the biotechnology sector, with the Department of Energy estimating ethanol usage could reduce U.S. petroleum-derived fuel consumption by up to 30% by 2030.에너지부는 에탄올 사용량이 2030년까지 미국의 석유 유래 연료 소비량을 최대 30%까지 줄일 수 있을 것으로 전망하고 있어 바이오 연료에 대한 수요 증가는 생명공학 분야에 희소식이 될 것으로 예상된다. The biotechnology sector has allowed the U.S. farming industry to rapidly increase its supply of corn and soybeans—the main inputs into biofuels—by developing genetically modified seeds that resist pests and drought. 생명공학 분야는 해충과 가뭄에 저항하는 유전자 변형 종자를 개발함으로써 미국 농업이 생물연료의 주요 투입물인 옥수수와 콩의 공급을 빠르게 늘릴 수 있도록 했다. By increasing farm productivity, biotechnology boosts biofuel production.[21] 농장의 생산성을 높임으로써, 생명공학은 생물연료 생산을 증가시킨다.[21]
Examples예[[edit편집]]

A rose plant that began as cells grown in a tissue culture조직 배양에서 세포가 자라면서 시작된 장미 식물
Biotechnology has applications in four major industrial areas, including health care (medical), crop production and agriculture, non-food (industrial) uses of crops and other products (e.g. biodegradable plastics, vegetable oil, biofuels), and environmental uses.생명공학은 의료(의료), 농작물 생산 및 농업, 농작물 및 기타 제품의 비식품(산업) 용도(생분해성 플라스틱, 식물성 기름, 바이오 연료), 환경 용도 등 4대 산업 분야에 응용이 가능하다.
For example, one application of biotechnology is the directed use of microorganisms for the manufacture of organic products (examples include beer and milk products).예를 들어, 생명공학의 한 가지 응용은 유기농 제품 제조에 미생물을 직접 사용하는 것이다(맥주와 우유 제품을 포함한다). Another example is using naturally present bacteria by the mining industry in bioleaching. 또 다른 예는 바이오 리어링에 광산업이 자연적으로 존재하는 박테리아를 사용하는 것이다. Biotechnology is also used to recycle, treat waste, clean up sites contaminated by industrial activities (bioremediation), and also to produce biological weapons. 생명공학은 재활용, 폐기물 처리, 산업활동(생물화)으로 오염된 현장 정화, 생물무기 생산에도 활용된다.
A series of derived terms have been coined to identify several branches of biotechnology, for example: 예를 들어 생명공학의 여러 분야를 식별하기 위해 일련의 파생된 용어들이 만들어졌다.
- Bioinformatics (also called "gold biotechnology") is an interdisciplinary field that addresses biological problems using computational techniques, and makes the rapid organization as well as analysis of biological data possible.생물정보학(bio informatics, "금 생명공학"이라고도 함)은 계산 기법을 이용하여 생물학적 문제를 해결하는 학제간 분야로, 생물학적 데이터의 분석은 물론 신속한 조직화가 가능하게 한다. The field may also be referred to as computational biology, and can be defined as, "conceptualizing biology in terms of molecules and then applying informatics techniques to understand and organize the information associated with these molecules, on a large scale."[22] 이 분야를 컴퓨터 생물학이라고도 할 수 있으며, "분자의 관점에서 생물학을 개념화한 다음 이러한 분자와 관련된 정보를 대규모로 이해하고 정리하기 위해 정보학 기법을 적용하는 것"[22]으로 정의할 수 있다. Bioinformatics plays a key role in various areas, such as functional genomics, structural genomics, and proteomics, and forms a key component in the biotechnology and pharmaceutical sector.[23] 생물정보학은 기능유전체학, 구조유전체학, 프로테오믹스 등 다양한 분야에서 핵심적인 역할을 하고 있으며, 생명공학 및 제약분야의 핵심요소를 형성하고 있다.[23]
- Blue biotechnology is based on the exploitation of sea resources to create products and industrial applications.[24]블루 바이오테크놀로지는 상품과 산업용 응용프로그램을 만들기 위한 해양자원의 착취에 기반을 두고 있다.[24] This branch of biotechnology is the most used for the industries of refining and combustion principally on the production of bio-oils with photosynthetic micro-algae.[24][25] 이 생명공학의 분과는 주로 광합성 미세조류를 이용한 생물오일 생산에 정제 및 연소 산업에 가장 많이 사용된다.[24][25]
- Green biotechnology is biotechnology applied to agricultural processes.녹색 생명공학은 농업 과정에 적용되는 생명공학이다. An example would be the selection and domestication of plants via micropropagation. 예를 들어 마이크로프로파게이션을 통해 식물을 선택하고 재배하는 것이 있을 것이다. Another example is the designing of transgenic plants to grow under specific environments in the presence (or absence) of chemicals. 또 다른 예는 화학물질이 존재하는(또는 없는) 특정 환경에서 성장하도록 유전자 변형 식물을 설계하는 것이다. One hope is that green biotechnology might produce more environmentally friendly solutions than traditional industrial agriculture. 한 가지 희망은 녹색 생명공학이 전통적인 산업 농업보다 더 환경 친화적인 해결책을 만들어 낼 수 있다는 것이다. An example of this is the engineering of a plant to express a pesticide, thereby ending the need of external application of pesticides. 그 예로 농약을 표현하기 위한 식물의 엔지니어링을 들 수 있으며, 따라서 농약의 외부적 적용의 필요성을 없앨 수 있다. An example of this would be Bt corn. 이것의 예는 Bt 옥수수일 것이다. Whether or not green biotechnology products such as this are ultimately more environmentally friendly is a topic of considerable debate.[24] 이와 같은 녹색 생명공학 제품이 궁극적으로 더 환경 친화적인지 아닌지는 상당한 논쟁거리가 되고 있다.[24] It is commonly considered as the next phase of green revolution, which can be seen as a platform to eradicate world hunger by using technologies which enable the production of more fertile and resistant, towards biotic and abiotic stress, plants and ensures application of environmentally friendly fertilizers and the use of biopesticides, it is mai 흔히 녹색혁명의 다음 단계로 여겨지는데, 보다 비옥하고 내성이 있는 생물의 생성을 가능하게 하는 기술, 식물, 친환경 비료의 적용과 생화학제의 사용을 보장하는 기술을 사용함으로써 세계 기아 근절을 위한 플랫폼으로 볼 수 있다.nly focused on the development of agriculture.[24]nly는 농업의 발전에 초점을 맞추었다.[24] On the other hand, some of the uses of green biotechnology involve microorganisms to clean and reduce waste.[26][24] 반면에, 녹색 생명공학의 사용의 일부는 폐기물을 청소하고 줄이기 위해 미생물을 포함한다.[26][24]
- Red biotechnology is the use of biotechnology in the medical and pharmaceutical industries, and health preservation.[24]적색 생명공학은 의료 및 제약 산업에서 생명공학을 사용하고, 건강을 보존하는 것이다.[24] This branch involves the production of vaccines and antibiotics, regenerative therapies, creation of artificial organs and new diagnostics of diseases.[24] 이 분과는 백신과 항생제, 재생요법, 인공장기의 생성, 질병의 새로운 진단 등을 포함한다.[24] As well as the development of hormones, stem cells, antibodies, siRNA and diagnostic tests.[24] 호르몬, 줄기세포, 항체, siRNA, 진단 테스트의 발달뿐만 아니라.[24]
- White biotechnology, also known as industrial biotechnology, is biotechnology applied to industrial processes.산업생명공학으로도 알려진 백색생명공학은 산업 과정에 적용되는 생명공학이다. An example is the designing of an organism to produce a useful chemical. 예를 들면 유용한 화학물질을 생산하기 위한 유기체의 설계가 있다. Another example is the using of enzymes as industrial catalysts to either produce valuable chemicals or destroy hazardous/polluting chemicals. 또 다른 예는 효소를 산업 촉매로 사용하여 귀중한 화학물질을 생산하거나 유해/공해 화학물질을 파괴하는 것이다. White biotechnology tends to consume less in resources than traditional processes used to produce industrial goods.[27][28] 백인 생명공학은 산업재료를 생산하는데 사용되는 전통적인 과정보다 자원을 덜 소비하는 경향이 있다.[27][28]
- "Yellow biotechnology" refers to the use of biotechnology in food production, for example in making wine, cheese, and beer by fermentation.[24]"노란 생명공학"은 식품 생산에 생명공학을 사용하는 것을 말하며, 예를 들어 발효에 의해 와인, 치즈, 맥주를 만드는 것을 말한다.[24] It has also been used to refer to biotechnology applied to insects. 곤충에 적용되는 생명공학을 지칭하는 용도로도 쓰였다. This includes biotechnology-based approaches for the control of harmful insects, the characterisation and utilisation of active ingredients or genes of insects for research, or application in agriculture and medicine and various other approaches.[29] 여기에는 해충의 통제를 위한 생명공학 기반 접근법, 연구를 위한 곤충의 활성 성분이나 유전자의 특성화 및 활용법, 또는 농업과 의학 및 기타 다양한 접근법이 포함된다.[29]
- Gray biotechnology is dedicated to environmental applications, and focused on the maintenance of biodiversity and the remotion of pollutants.[24]회색 생명공학은 환경 응용에 전념하고 있으며 생물 다양성의 유지와 오염물질의 이동에 초점을 맞추고 있다.[24]
- Brown biotechnology is related to the management of arid lands and deserts.갈색 생명공학은 건조한 땅과 사막의 관리와 관련이 있다. One application is the creation of enhanced seeds that resist extreme environmental conditions of arid regions, which is related to the innovation, creation of agriculture techniques and management of resources.[24] 한 가지 적용은 건조 지역의 극한 환경 조건에 저항하는 강화된 종자를 만드는 것인데, 이것은 혁신, 농업 기법의 창조, 자원 관리와 관련이 있다.[24]
- Violet biotechnology is related to law, ethical and philosophical issues around biotechnology.[24]바이올렛 생명공학은 생명공학과 관련된 법, 윤리적, 철학적 문제와 관련이 있다.[24]
- Dark biotechnology is the color associated with bioterrorism or biological weapons and biowarfare which uses microorganisms, and toxins to cause diseases and death in humans, livestock and crops.[30][24]다크 바이오테크놀로지는 생물학적 오류주의 또는 생물학적 무기 및 미생물을 이용하는 바이오와르페어와 관련된 색이며, 독소를 이용하여 인간, 가축, 농작물에 질병과 죽음을 초래한다.[30][24]
Medicine의학[[edit편집]]
In medicine, modern biotechnology has many applications in areas such as pharmaceutical drug discoveries and production, pharmacogenomics, and genetic testing (or genetic screening).의학에서 현대 생명공학은 제약의약품 발견과 생산, 약리유전학, 유전자 검사(또는 유전자 검사)와 같은 분야에서 많은 응용을 하고 있다.

DNA microarray chip – some can do as many as a million blood tests at onceDNA 마이크로 어레이 칩 – 어떤 칩은 한 번에 100만 개에 달하는 혈액 검사를 수행할 수 있음
Pharmacogenomics (a combination of pharmacology and genomics) is the technology that analyses how genetic makeup affects an individual's response to drugs.[31]약리유전체학(약리학과 유전체의 조합)은 유전적 구성이 약물에 대한 개인의 반응에 어떻게 영향을 미치는지 분석하는 기술이다.[31] Researchers in the field investigate the influence of genetic variation on drug responses in patients by correlating gene expression or single-nucleotide polymorphisms with a drug's efficacy or toxicity.[32] 이 분야의 연구자들은 유전자 발현이나 단일 뉴클레오티드 다형성과 약물의 효능이나 독성을 연관시켜 유전적 변화가 환자의 약물 반응에 미치는 영향을 연구한다.[32] The purpose of pharmacogenomics is to develop rational means to optimize drug therapy, with respect to the patients' genotype, to ensure maximum efficacy with minimal adverse effects.[33] 약리유전체학의 목적은 환자의 유전자형에 관해서 약물 치료를 최적화하기 위한 합리적인 수단을 개발하여 최소한의 부작용만으로 최대의 효과를 보장하는 것이다.[33] Such approaches promise the advent of "personalized medicine"; in which drugs and drug combinations are optimized for each individual's unique genetic makeup.[34][35] 이러한 접근법은 약물과 약물 조합이 각 개인의 독특한 유전자 구성에 최적화되어 있는 "개인화된 의학"의 출현을 약속한다.[34][35]
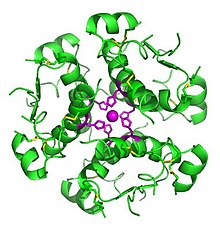
Computer-generated image of insulin hexamers highlighting the threefold symmetry, the zinc ions holding it together, and the histidine residues involved in zinc binding3배 대칭, 아연 이온, 아연 결합과 관련된 히스티딘 잔류물을 강조하는 인슐린 헥사머의 컴퓨터 생성 이미지
Biotechnology has contributed to the discovery and manufacturing of traditional small molecule pharmaceutical drugs as well as drugs that are the product of biotechnology – biopharmaceutics.생명공학은 생명공학의 산물인 약뿐만 아니라 전통적인 소분자 의약품의 발견과 제조에 기여했다. Modern biotechnology can be used to manufacture existing medicines relatively easily and cheaply. 현대의 생명공학은 기존 의약품을 비교적 쉽고 저렴하게 제조하는데 이용될 수 있다. The first genetically engineered products were medicines designed to treat human diseases. 최초의 유전자 조작 제품은 인간의 질병을 치료하기 위해 고안된 약이었다. To cite one example, in 1978 Genentech developed synthetic humanized insulin by joining its gene with a plasmid vector inserted into the bacterium Escherichia coli. 한 가지 예를 들어, 1978년 제넨텍은 대장균 박테리아에 삽입된 플라스미드 벡터와 결합하여 합성 인간화된 인슐린을 개발했다. Insulin, widely used for the treatment of diabetes, was previously extracted from the pancreas of abattoir animals (cattle or pigs). 당뇨병 치료에 널리 사용되는 인슐린은 이전에 췌장동물(고양이 또는 돼지)에서 추출한 것이다. The genetically engineered bacteria are able to produce large quantities of synthetic human insulin at relatively low cost.[36][37] 유전자 조작 박테리아는 비교적 저렴한 비용으로 많은 양의 합성 인간 인슐린을 생산할 수 있다.[36][37] Biotechnology has also enabled emerging therapeutics like gene therapy. 생명공학은 또한 유전자 치료와 같은 새로운 치료법을 가능하게 했다. The application of biotechnology to basic science (for example through the Human Genome Project) has also dramatically improved our understanding of biology and as our scientific knowledge of normal and disease biology has increased, our ability to develop new medicines to treat previously untreatable diseases has increased as well.[37] 기초과학에 생명공학을 응용한 것(예를 들어 인간 게놈 프로젝트를 통해)도 생물학에 대한 우리의 이해를 획기적으로 향상시켰고, 정상 생물학과 질병 생물학에 대한 우리의 과학적 지식이 증가함에 따라 이전에 치료할 수 없었던 질병을 치료하기 위한 신약 개발 능력도 함께 증가했다.[37]
Genetic testing allows the genetic diagnosis of vulnerabilities to inherited diseases, and can also be used to determine a child's parentage (genetic mother and father) or in general a person's ancestry.유전자 검사는 유전적 질병에 대한 취약성의 유전적 진단을 가능하게 하며, 아이의 부모(유전적 어머니와 아버지) 또는 일반적으로 사람의 조상을 결정하는 데도 사용될 수 있다. In addition to studying chromosomes to the level of individual genes, genetic testing in a broader sense includes biochemical tests for the possible presence of genetic diseases, or mutant forms of genes associated with increased risk of developing genetic disorders. 개별 유전자의 수준으로 염색체를 연구하는 것 외에도, 보다 넓은 의미의 유전자 검사는 유전 질환의 존재 가능성에 대한 생화학적 검사나 유전 질환의 발생 위험 증가와 관련된 돌연변이 형태의 유전자를 포함한다. Genetic testing identifies changes in chromosomes, genes, or proteins.[38] 유전자 검사는 염색체, 유전자, 단백질의 변화를 확인한다.[38] Most of the time, testing is used to find changes that are associated with inherited disorders. 대부분의 경우, 검사는 유전적 장애와 관련된 변화를 찾기 위해 사용된다. The results of a genetic test can confirm or rule out a suspected genetic condition or help determine a person's chance of developing or passing on a genetic disorder. 유전자 검사 결과는 의심스러운 유전적 상태를 확인하거나 배제하거나 유전적 질환이 발병하거나 유전적 질환이 전이될 가능성을 판단하는 데 도움을 줄 수 있다. As of 2011 several hundred genetic tests were in use.[39][40] 2011년 현재 수백 건의 유전자 검사가 사용되고 있다.[39][40] Since genetic testing may open up ethical or psychological problems, genetic testing is often accompanied by genetic counseling. 유전자 검사는 윤리적 또는 심리적 문제를 일으킬 수 있기 때문에 유전자 검사는 종종 유전자 상담을 동반한다.
Agriculture농업[[edit편집]]
Genetically modified crops ("GM crops", or "biotech crops") are plants used in agriculture, the DNA of which has been modified with genetic engineering techniques.유전자 변형 작물("GM 작물" 또는 "바이오텍 작물")은 농업에 사용되는 식물이며, 그 DNA는 유전공학 기술로 변형되었다. In most cases, the main aim is to introduce a new trait that does not occur naturally in the species. 대부분의 경우 종에서 자연적으로 발생하지 않는 새로운 특성을 도입하는 것이 주된 목적이다. Biotechnology firms can contribute to future food security by improving the nutrition and viability of urban agriculture. 생명공학 회사들은 도시 농업의 영양과 생존 가능성을 향상시킴으로써 미래의 식량 안보에 기여할 수 있다. Furthermore, the protection of intellectual property rights encourages private sector investment in agrobiotechnology. 더욱이 지적재산권의 보호는 민간부문에서 농업기술에 대한 투자를 장려한다. For example, in Illinois FARM Illinois (Food and Agriculture RoadMap for Illinois) is an initiative to develop and coordinate farmers, industry, research institutions, government, and nonprofits in pursuit of food and agriculture innovation. 예를 들어 일리노이주 FARM 일리노이주(Illinois for Illinois)에서는 식량과 농업 혁신을 추구하기 위해 농업인, 산업, 연구기관, 정부, 비영리단체를 개발하고 조정하는 이니셔티브가 있다. In addition, the Illinois Biotechnology Industry Organization (iBIO) is a life sciences industry association with more than 500 life sciences companies, universities, academic institutions, service providers and others as members. 또 일리노이 생명공학산업기구(iBIO)는 생명과학 기업, 대학, 학술기관, 서비스 제공업체 등 500여 곳이 회원으로 가입돼 있는 생명과학산업협회다. The association describes its members as "dedicated to making Illinois and the surrounding Midwest one of the world’s top life sciences centers."[41] 이 협회는 회원들을 "일리노이와 주변 중서부를 세계 최고의 생명과학 센터로 만드는 데 헌신하고 있다"[41]고 설명했다.
Examples in food crops include resistance to certain pests,[42] diseases,[43] stressful environmental conditions,[44] resistance to chemical treatments (e.g. resistance to a herbicide[45]), reduction of spoilage,[46] or improving the nutrient profile of the crop.[47]식품 작물의 예로는 특정 해충에 대한 저항성,[42] 질병,[43] 스트레스 받는 환경 조건,[44] 화학적 치료에 대한 저항성(예[45]: 제초제에 대한 저항성), 부패의 감소 [46]또는 작물의 영양학적 측면 개선 등이 있다.[47] Examples in non-food crops include production of pharmaceutical agents,[48] biofuels,[49] and other industrially useful goods,[50] as well as for bioremediation.[51][52] 비식품 작물의 예로는 바이오 연료 [48][49]및 기타 산업적으로 유용한 물품의 생산과 생물 거품 제조를 들 수 있다.[50][51][52]
Farmers have widely adopted GM technology.농부들은 GM 기술을 널리 채택해 왔다. Between 1996 and 2011, the total surface area of land cultivated with GM crops had increased by a factor of 94, from 17,000 square kilometers (4,200,000 acres) to 1,600,000 km2 (395 million acres).[53] 1996년과 2011년 사이에 GM 작물로 경작된 토지의 총 지표면적은 1만7천 평방 킬로미터(420만 에이커)에서 160만 km2(3억9천 5백만 에이커)로 94배 증가했다.[53] 10% of the world's crop lands were planted with GM crops in 2010.[53] 2010년 세계 농작물의 10%가 GM 작물로 심어졌다.[53] As of 2011, 11 different transgenic crops were grown commercially on 395 million acres (160 million hectares) in 29 countries such as the US, Brazil, Argentina, India, Canada, China, Paraguay, Pakistan, South Africa, Uruguay, Bolivia, Australia, Philippines, Myanmar, Burkina Faso, Mexico and Spain.[53] 2011년 기준으로 미국, 브라질, 아르헨티나, 인도, 캐나다, 중국, 파라과이, 파키스탄, 남아프리카공화국, 우루과이, 볼리비아, 호주, 필리핀, 미얀마, 부르키나파소, 멕시코, 스페인 등 29개국에서 3억9500만 에이커(1억6,000만 헥타르)에 11종의 유전자 변형 작물이 상업적으로 재배되었다.[53]
Genetically modified foods are foods produced from organisms that have had specific changes introduced into their DNA with the methods of genetic engineering.유전자변형식품은 유전공학의 방법으로 DNA에 특정한 변화를 가져온 유기체로부터 생산된 식품이다. These techniques have allowed for the introduction of new crop traits as well as a far greater control over a food's genetic structure than previously afforded by methods such as selective breeding and mutation breeding.[54] 이러한 기법들은 새로운 작물 형질을 도입할 뿐만 아니라 음식의 유전적 구조에 대한 통제도 선택적 사육과 돌연변이 사육과 같은 방법들에 의해 이전에 제공되었던 것보다 훨씬 더 크게 허용되었다.[54] Commercial sale of genetically modified foods began in 1994, when Calgene first marketed its Flavr Savr delayed ripening tomato.[55] 유전자 변형 식품의 상업적 판매는 1994년에 시작되었는데, 그 때 Calgene이 그것의 플라브르 사브르를 처음 시판하면서 토마토 숙성을 지연시켰다.[55] To date most genetic modification of foods have primarily focused on cash crops in high demand by farmers such as soybean, corn, canola, and cotton seed oil. 현재까지 대부분의 식품 유전자변형은 주로 콩, 옥수수, 카놀라, 목화씨유와 같은 농부들의 수요가 높은 현금성 작물에 초점을 맞추고 있다. These have been engineered for resistance to pathogens and herbicides and better nutrient profiles. 이것들은 병원균과 제초제에 대한 저항성과 더 나은 영양소 프로파일링을 위해 설계되었다. GM livestock have also been experimentally developed; in November 2013 none were available on the market,[56] but in 2015 the FDA approved the first GM salmon for commercial production and consumption.[57] GM 가축도 실험적으로 개발되었다; 2013년 11월에 시장에 나온 연어는 없었지만,[56] FDA는 2015년에 상업적 생산과 소비를 위한 최초의 GM 연어를 승인했다.[57]
There is a scientific consensus[58][59][60][61] that currently available food derived from GM crops poses no greater risk to human health than conventional food,[62][63][64][65][66] but that each GM food needs to be tested on a case-by-case basis before introduction.[67][68][69]현재 GM 작물에서 파생된 가용 식품은 재래식 식품보다 인간의 건강에 더 큰 위험을 주지는 않지만,[62][63][64][65][66] 각각의 GM 식품은 도입 전에 사례별로 시험할 필요가 있다는[58][59][60][61] 과학적 공감대가 있다.[67][68][69] Nonetheless, members of the public are much less likely than scientists to perceive GM foods as safe.[70][71][72][73] 그럼에도 불구하고, 일반 대중들은 유전자 조작 식품이 안전하다고 생각하는 과학자들보다 훨씬 덜하다.[70][71][72][73] The legal and regulatory status of GM foods varies by country, with some nations banning or restricting them, and others permitting them with widely differing degrees of regulation.[74][75][76][77] GM 식품의 법적, 규제적 상태는 국가별로 다르며, 일부 국가는 금지 또는 제한하고, 다른 국가는 규제 정도가 크게 다른 것을 허용한다.[74][75][76][77]
GM crops also provide a number of ecological benefits, if not used in excess.[78]GM 작물은 또한 과도하게 사용하지 않을 경우 많은 생태학적 혜택을 제공한다.[78] However, opponents have objected to GM crops per se on several grounds, including environmental concerns, whether food produced from GM crops is safe, whether GM crops are needed to address the world's food needs, and economic concerns raised by the fact these organisms are subject to intellectual property law. 그러나 반대론자들은 환경적 우려, GM 작물에서 생산된 식품이 안전한지 여부, 세계의 식량 수요를 해결하기 위해 GM 작물이 필요한지 여부, 그리고 이러한 유기체들이 지적 재산법의 적용을 받는다는 사실에 의해 제기되는 경제적 우려 등 몇 가지 이유로 GM 작물에 반대해 왔다.
Industrial공업[[edit편집]]
Industrial biotechnology (known mainly in Europe as white biotechnology) is the application of biotechnology for industrial purposes, including industrial fermentation.산업생명공학(주로 유럽에서는 백색생명공학으로 알려져 있다)은 산업 발효를 포함한 산업목적에 대한 생명공학의 응용이다. It includes the practice of using cells such as microorganisms, or components of cells like enzymes, to generate industrially useful products in sectors such as chemicals, food and feed, detergents, paper and pulp, textiles and biofuels.[79] 화학, 식품 및 사료, 세제, 종이 및 펄프, 직물, 바이오 연료 등의 분야에서 산업적으로 유용한 제품을 생산하기 위해 미생물 등의 세포나 효소 같은 세포 성분을 사용하는 관행이 포함된다.[79] In the current decades, significant progress has been done in creating genetically modified organisms (GMOs) that enhance the diversity of applications and economical viability of industrial biotechnology. 현재 수십 년 동안, 산업 생명공학의 응용의 다양성과 경제적 생존 가능성을 향상시키는 유전자 변형 유기체(GMO)를 만드는 데 상당한 진전이 있었다. By using renewable raw materials to produce a variety of chemicals and fuels, industrial biotechnology is actively advancing towards lowering greenhouse gas emissions and moving away from a petrochemical-based economy.[80] 재생 가능한 원료를 사용하여 다양한 화학 물질과 연료를 생산함으로써, 산업 생명공학은 온실 가스 배출량을 줄이고 석유화학 기반의 경제에서 벗어나는 방향으로 활발하게 나아가고 있다.[80]
Environmental환경[[edit편집]]
The environment can be affected by biotechnologies, both positively and adversely.환경은 생물 공학에 의해 긍정적으로나 나쁘게나 영향을 받을 수 있다. Vallero and others have argued that the difference between beneficial biotechnology (e.g.bioremediation is to clean up an oil spill or hazard chemical leak) versus the adverse effects stemming from biotechnological enterprises (e.g. flow of genetic material from transgenic organisms into wild strains) can be seen as applications and implications, Vallero와 다른 이들은 유익한 생명공학(예: 생물학적 매개체는 기름 유출이나 유해화학물질 누출 청소)과 생명공학 기업에서 야기되는 부작용(예: 유전자 변형 유기체에서 야생 변종으로 유전적 물질의 흐름)의 차이를 응용과 함의로 볼 수 있다고 주장해왔다. respectively.[81]각각[81] Cleaning up environmental wastes is an example of an application of environmental biotechnology; whereas loss of biodiversity or loss of containment of a harmful microbe are examples of environmental implications of biotechnology. 환경 폐기물을 청소하는 것은 환경 생명공학의 적용 사례인 반면, 생물다양성의 상실이나 유해한 미생물 봉쇄의 상실은 생명공학의 환경적 영향의 예다.
Regulation규정[[edit편집]]
Main articles:주요 기사: Regulation of genetic engineering and Regulation of the release of genetic modified organisms 유전공학 규정 및 유전자 변형 유기체 방류 규정
The regulation of genetic engineering concerns approaches taken by governments to assess and manage the risks associated with the use of genetic engineering technology, and the development and release of genetically modified organisms (GMO), including genetically modified crops and genetically modified fish.유전공학에 관한 규제는 정부가 유전공학 기술의 사용과 관련된 위험과 유전자변형 작물 및 유전자변형 어류를 포함한 유전자변형생물(GMO)의 개발과 방출을 평가하고 관리하기 위해 취하는 접근법이다. There are differences in the regulation of GMOs between countries, with some of the most marked differences occurring between the US and Europe.[82] 국가 간 GMO 규제에 차이가 있는데, 미국과 유럽 간에는 가장 두드러진 차이가 일부 발생한다.[82] Regulation varies in a given country depending on the intended use of the products of the genetic engineering. 규제는 특정 국가에서 유전공학 제품의 의도된 사용에 따라 다르다. For example, a crop not intended for food use is generally not reviewed by authorities responsible for food safety.[83] 예를 들어, 식품 사용을 의도하지 않은 작물은 일반적으로 식품 안전을 책임지는 당국에 의해 검토되지 않는다.[83] The European Union differentiates between approval for cultivation within the EU and approval for import and processing. 유럽연합은 EU 내의 경작 승인과 수입과 가공 승인 사이에서 구별된다. While only a few GMOs have been approved for cultivation in the EU a number of GMOs have been approved for import and processing.[84] EU에서 경작 승인을 받은 GMO는 몇 개에 불과하지만, 수입과 가공 승인을 받은 GMO도 적지 않다.[84] The cultivation of GMOs has triggered a debate about coexistence of GM and non GM crops. GMO의 재배는 GM과 비GM 작물의 공존에 대한 논쟁을 촉발시켰다. Depending on the coexistence regulations, incentives for cultivation of GM crops differ.[85] 상생규제에 따라 GM 작물 재배에 따른 인센티브가 달라진다.[85]
Learning배우는 것은[[edit편집]]
In 1988, after prompting from the United States Congress, the National Institute of General Medical Sciences (National Institutes of Health) (NIGMS) instituted a funding mechanism for biotechnology training.1988년, 미국 의회에서 촉구한 후, 국립 종합 의학 연구소(National Institute of Health)는 생명공학 훈련을 위한 기금 메커니즘을 설립했다. Universities nationwide compete for these funds to establish Biotechnology Training Programs (BTPs). 전국의 대학들은 생명공학 훈련 프로그램을 설립하기 위해 이 기금을 위해 경쟁한다. Each successful application is generally funded for five years then must be competitively renewed. 성공적인 각 신청서는 일반적으로 5년간 자금을 지원받은 후 경쟁적으로 갱신되어야 한다. Graduate students in turn compete for acceptance into a BTP; if accepted, then stipend, tuition and health insurance support is provided for two or three years during the course of their Ph.D. thesis work. 대학원생들은 차례로 BTP로 합격하기 위해 경쟁하며, 합격하면 박사 논문 작업 과정에서 2, 3년간 등록금과 건강보험 지원이 제공된다. Nineteen institutions offer NIGMS supported BTPs.[86] 19개 기관은 NIGMS가 지원하는 BTP를 제공한다.[86] Biotechnology training is also offered at the undergraduate level and in community colleges. 생명공학 교육은 학부 수준과 지역사회 대학에서도 제공된다.
References and notes참조 및 참고 사항[[edit편집]]
- ^^ Biotechnology Archived November 7, 2012, at the Wayback Machine.생명공학은 2012년 11월 7일 웨이백 기계에 보관되었다. Portal.acs.org. Portal.acs.org. Retrieved on March 20, 2013. 2013년 3월 20일에 검색됨
- ^^ .mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"\"""\"""'""'"}.mw-parser-output .id-lock-free a,.mw-parser-output .citation .cs1-lock-free a{background:linear-gradient(transparent,transparent),url("//upload.wikimedia.org/wikipedia/commons/6/65/Lock-green.svg")right 0.1em center/9px no-repeat}.mw-parser-output .id-lock-limited a,.mw-parser-output .id-lock-registration a,.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:linear-gradient(transparent,transparent),url("//upload.wikimedia.org/wikipedia/commons/d/d6/Lock-gray-alt-2.svg")right 0.1em center/9px no-repeat}.mw-parser-output .id-lock-subscription a,.mw-parser-output .citation .cs1-lock-subscription a{background:linear-gradient(transparent,transparent),url("//upload.wikimedia.org/wikipedia/commons/a/aa/Lock-red-alt-2.svg")right 0.1em center/9px no-repeat}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:linear-gradient(transparent,transparent),url("//upload.wikimedia.org/wikipedia/commons/4/4c/Wikisource-logo.svg")right 0.1em center/12px no-repeat}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:none;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}.mw-parser-output .citation .mw-selflink{font-weight:inherit} "Archived copy" (PDF). Archived from the original (PDF) on August 7, 2015. Retrieved December 29, 2014.CS1 maint: archived copy as title (link)CS1 maint: 제목으로 보관된 복사본(링크)
- ^^ What is biotechnology?.생명공학이란 무엇인가? Europabio. 유로파비오. Retrieved on March 20, 2013. 2013년 3월 20일에 검색됨
- ^^ Key Biotechnology Indicators (December 2011).주요 생명공학 지표(2011년 12월) oecd.org oecd.org
- ^^ Biotechnology policies – Organization for Economic Co-operation and Development.생명공학 정책 – 경제 협력 개발 기구 Oecd.org. Oecd.org. Retrieved on March 20, 2013. 2013년 3월 20일에 검색됨
- ^^ "History, scope and development of biotechnology". iopscience.iop.org. Retrieved October 30, 2018.
- ^^ What Is Bioengineering?생명공학이란 무엇인가? Archived January 23, 2013, at the Wayback Machine. 2013년 1월 23일 웨이백 머신에 보관. Bionewsonline.com. Bionewsonline.com. Retrieved on March 20, 2013. 2013년 3월 20일에 검색됨
- ^^ See Arnold JP (2005). Origin and History of Beer and Brewing: From Prehistoric Times to the Beginning of Brewing Science and Technology. Cleveland, Ohio: BeerBooks. p. 34. ISBN 978-0-9662084-1-2. OCLC 71834130..참조.
- ^^ Cole-Turner R (2003). "Biotechnology". Encyclopedia of Science and Religion. Retrieved December 7, 2014.
- ^ Jump up to: a b^ 위로 이동: Thieman WJ, Palladino MA (2008). Introduction to Biotechnology. Pearson/Benjamin Cummings. ISBN 978-0-321-49145-9.
- ^^ Springham D, Springham G, Moses V, Cape RE (1999). Biotechnology: The Science and the Business. CRC Press. p. 1. ISBN 978-90-5702-407-8.
- ^^ "Diamond v. Chakrabarty, 447 U.S. 303 (1980)."다이아몬드 대 차크라바티 사건, 447 U.S. 303(1980년). No. 79-139." 79-139번." United States Supreme Court. 미국 대법원. June 16, 1980. 1980년 6월 16일. Retrieved on May 4, 2007. 2007년 5월 4일에 검색됨.
- ^^ "1960: Metal Oxide Semiconductor (MOS) Transistor Demonstrated". The Silicon Engine: A Timeline of Semiconductors in Computers. Computer History Museum. Retrieved August 31, 2019.
- ^^ Park, Jeho; Nguyen, Hoang Hiep; Woubit, Abdela; Kim, Moonil (2014). "Applications of Field-Effect Transistor (FET)–Type Biosensors". Applied Science and Convergence Technology. 23 (2): 61–71. doi:10.5757/ASCT.2014.23.2.61. ISSN 2288-6559. S2CID 55557610.
- ^^ Clark, Leland C.; Lyons, Champ (1962). "Electrode Systems for Continuous Monitoring in Cardiovascular Surgery". Annals of the New York Academy of Sciences. 102 (1): 29–45. Bibcode:1962NYASA.102...29C. doi:10.1111/j.1749-6632.1962.tb13623.x. ISSN 1749-6632. PMID 14021529.
- ^ Jump up to: a b c^ 위로 이동: Bergveld, Piet (October 1985). "The impact of MOSFET-based sensors" (PDF). Sensors and Actuators. 8 (2): 109–127. Bibcode:1985SeAc....8..109B. doi:10.1016/0250-6874(85)87009-8. ISSN 0250-6874.
- ^^ Chris Toumazou; Pantelis Georgiou (December 2011). "40 years of ISFET technology:From neuronal sensing to DNA sequencing". Electronics Letters. Retrieved May 13, 2016.
- ^^ Bergveld, P. (January 1970). "Development of an Ion-Sensitive Solid-State Device for Neurophysiological Measurements". IEEE Transactions on Biomedical Engineering. BME-17 (1): 70–71. doi:10.1109/TBME.1970.4502688. PMID 5441220.
- ^ Jump up to: a b c^ 위로 이동: Schöning, Michael J.; Poghossian, Arshak (September 10, 2002). "Recent advances in biologically sensitive field-effect transistors (BioFETs)" (PDF). Analyst. 127 (9): 1137–1151. Bibcode:2002Ana...127.1137S. doi:10.1039/B204444G. ISSN 1364-5528. PMID 12375833.
- ^^ VoIP Providers And Corn Farmers Can Expect To Have Bumper Years In 2008 And Beyond, According To The Latest Research Released By Business Information Analysts At IBISWorld.IBISWold의 기업 정보 분석가가 최근 발표한 연구에 따르면 VoIP 제공업체와 옥수수 농가는 2008년과 그 이후까지 풍년이 들 것으로 예상된다. Los Angeles (March 19, 2008) 로스앤젤레스 (2008년 3월 19일)
- ^^ "The Recession List - Top 10 Industries to Fly and Flop in 2008". Bio-Medicine.org. March 19, 2008.
- ^^ Gerstein, M. "Bioinformatics Introduction Archived 2007-06-16 at the Wayback Machine."게르슈타인, M. "생물정보학 입문서 2007-06-16 웨이백 머신에 보관" Yale University. 예일 대학교. Retrieved on May 8, 2007. 2007년 5월 8일에 검색됨.
- ^^ Siam, R. (2009).Siam, R. (2009년). Biotechnology Research and Development in Academia: providing the foundation for Egypt's Biotechnology spectrum of colors. 학계의 생명공학 연구 개발: 이집트의 생명공학 스펙트럼의 색상을 위한 기초를 제공한다. Sixteenth Annual American University in Cairo Research Conference, American University in Cairo, Cairo, Egypt. 이집트 카이로 카이로의 16번째 미국 대학, 카이로의 미국 대학 연구 회의. BMC Proceedings, 31–35. BMC 프로시저 31-35
- ^ Jump up to: a b c d e f g h i j k l m^ 위로 이동: Kafarski, P. (2012).카파스키, P. (2012) Rainbow Code of Biotechnology. 레인보우 생명공학 강령 CHEMIK. Wroclaw University 케미크. 브로클로 대학교
- ^^ Biotech: true colours.생명공학: 진정한 색깔. (2009). (2009). TCE: The Chemical Engineer, (816), 26–31. TCE: 화학 엔지니어, (816), 26–31.
- ^^ Aldridge, S. (2009).알드리지, S. (2009) The four colours of biotechnology: the biotechnology sector is occasionally described as a rainbow, with each sub sector having its own colour. 생명공학의 네 가지 색깔: 생명공학 분야는 때때로 무지개로 묘사되며, 각 하위 섹터는 고유의 색을 가지고 있다. But what do the different colours of biotechnology have to offer the pharmaceutical industry. 하지만 생명공학의 다른 색깔들이 제약 산업에 제공할 수 있는 것은 무엇인가? Pharmaceutical Technology Europe, (1). 12. 제약 기술 유럽, (1). 12.
- ^^ Frazzetto G (September 2003). "White biotechnology". EMBO Reports. 4 (9): 835–7. doi:10.1038/sj.embor.embor928. PMC 1326365. PMID 12949582.
- ^^ Frazzetto, G. (2003).Frazzetto, G.(2003). White biotechnology. 화이트 바이오테크놀로지 March 21, 2017, de EMBOpress Sitio 2017년 3월 21일, 데 EMBOpress Sitio.
- ^^ Advances in Biochemical Engineering/Biotechnology, Volume 135 2013, Yellow Biotechnology I생화학공학/생명공학 발전, 135권 2013, 황색생명공학 I
- ^^ Edgar, J.D. (2004).에드거, J.D. (2004) The Colours of Biotechnology: 생명공학의 색깔: Science, Development and Humankind. 과학, 개발, 그리고 인류. Electronic Journal of Biotechnology, (3), 01 생명공학 전자저널, (3), 01
- ^^ Ermak G. (2013) Modern Science & Future Medicine (second edition)에르막 G. (2013) 모던 사이언스 & 퓨처 메디컬 (제2판)
- ^^ Wang L (2010). "Pharmacogenomics: a systems approach". Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 2 (1): 3–22. doi:10.1002/wsbm.42. PMC 3894835. PMID 20836007.
- ^^ Becquemont L (June 2009). "Pharmacogenomics of adverse drug reactions: practical applications and perspectives". Pharmacogenomics. 10 (6): 961–9. doi:10.2217/pgs.09.37. PMID 19530963.
- ^^ "Guidance for Industry Pharmacogenomic Data Submissions" (PDF). U.S. Food and Drug Administration. March 2005. Retrieved August 27, 2008.
- ^^ Squassina A, Manchia M, Manolopoulos VG, Artac M, Lappa-Manakou C, Karkabouna S, Mitropoulos K, Del Zompo M, Patrinos GP (August 2010). "Realities and expectations of pharmacogenomics and personalized medicine: impact of translating genetic knowledge into clinical practice". Pharmacogenomics. 11 (8): 1149–67. doi:10.2217/pgs.10.97. PMID 20712531.
- ^^ Bains W (1987). Genetic Engineering For Almost Everybody: What Does It Do? What Will It Do?. Penguin. p. 99. ISBN 978-0-14-013501-5.
- ^ Jump up to: a b^ 위로 이동: U.S. Department of State International Information Programs, "Frequently Asked Questions About Biotechnology", USIS Online; available from USinfo.state.gov Archived September 12, 2007, at the Wayback Machine, accessed September 13, 2007.미국 국무성 국제 정보 프로그램인 USIS 온라인, "생명공학에 대해 자주 묻는 질문"; 2007년 9월 12일 웨이백 머신에서 보관된 USinfo.state.gov에서 이용가능하며 2007년 9월 13일에 접속했다. Cf. Feldbaum C (February 2002). "Biotechnology. Some history should be repeated". Science. 295 (5557): 975. doi:10.1126/science.1069614. PMID 11834802. S2CID 32595222. cf.
- ^^ "What is genetic testing? – Genetics Home Reference". Ghr.nlm.nih.gov. May 30, 2011. Retrieved June 7, 2011.
- ^^ "Genetic Testing: MedlinePlus". Nlm.nih.gov. Retrieved June 7, 2011.
- ^^ "Definitions of Genetic Testing". Definitions of Genetic Testing (Jorge Sequeiros and Bárbara Guimarães). EuroGentest Network of Excellence Project. September 11, 2008. Archived from the original on February 4, 2009. Retrieved August 10, 2008.
- ^^ Mazany, Terry (May 19, 2015). "A FOOD AND AGRICULTURE ROADMAP FOR ILLINOIS" (PDF). learnbioscience.com/blog.
- ^^ Genetically Altered Potato Ok'd For Crops Lawrence Journal-World – May 6, 1995유전자변형감자 로렌스 저널 월드 (Genetic Changed For Crops Lawrence Journal-World
- ^^ National Academy of Sciences (2001). Transgenic Plants and World Agriculture. Washington: National Academy Press.
- ^^ Paarlburg R (January 2011). "Drought Tolerant GMO Maize in Africa, Anticipating Regulatory Hurdles" (PDF). International Life Sciences Institute. Archived from the original (PDF) on December 22, 2014. Retrieved April 25, 2011.
- ^^ Carpenter J. & Gianessi L. (1999).카펜터 J. & 지아네시 L. (1999년) Herbicide tolerant soybeans: 제초제 내성 콩: Why growers are adopting Roundup Ready varieties. 재배자들이 라운드업 레디 품종을 채택하는 이유 AgBioForum, 2(2), 65–72. AgBioForum, 2(2), 65–72.
- ^^ Haroldsen VM, Paulino G, Chi-ham C, Bennett AB (2012). "Research and adoption of biotechnology strategies could improve California fruit and nut crops" (PDF). California Agriculture. 66 (2): 62–69. doi:10.3733/ca.v066n02p62. Archived from the original (PDF) on May 11, 2013.
- ^^ About Golden Rice Archived November 2, 2012, at the Wayback Machine.2012년 11월 2일 웨이백머신에 보관된 황금 쌀 정보. Irri.org. Irri.org. Retrieved on March 20, 2013. 2013년 3월 20일에 검색됨
- ^^ Gali Weinreb and Koby Yeshayahou for Globes May 2, 2012.Globes의 Gali Weinreb와 Koby Yeshayahou는 2012년 5월 2일. FDA approves Protalix Gaucher treatment Archived May 29, 2013, at the Wayback Machine FDA는 2013년 5월 29일 웨이백 머신에 보관된 프로탈릭스 가우처 치료제를 승인한다.
- ^^ Carrington, Damien (January 19, 2012) GM microbe breakthrough paves way for large-scale seaweed farming for biofuels The Guardian.Carrington, Damien (2012년 1월 19일) GM microbe 돌파구는 The Guardian을 위한 대규모 미역 양식장을 위한 길을 열어준다. Retrieved March 12, 2012 2012년 3월 12일 검색됨
- ^^ van Beilen JB, Poirier Y (May 2008). "Production of renewable polymers from crop plants". The Plant Journal. 54 (4): 684–701. doi:10.1111/j.1365-313X.2008.03431.x. PMID 18476872. S2CID 25954199.
- ^^ Strange, Amy (September 20, 2011) Scientists engineer plants to eat toxic pollution The Irish Times.이상해, 에이미(2011년 9월 20일 ~ ) 과학자들은 아일랜드 타임즈를 먹기 위해 식물을 조작한다. Retrieved September 20, 2011 2011년 9월 20일 회수
- ^^ Diaz E (editor). (2008). Microbial Biodegradation: Genomics and Molecular Biology (1st ed.). Caister Academic Press. ISBN 978-1-904455-17-2.
- ^ Jump up to: a b c^ 위로 이동: James C (2011). "ISAAA Brief 43, Global Status of Commercialized Biotech/GM Crops: 2011". ISAAA Briefs. Ithaca, New York: International Service for the Acquisition of Agri-biotech Applications (ISAAA). Retrieved June 2, 2012.
- ^^ GM Science Review First Report Archived October 16, 2013, at the Wayback Machine, Prepared by the UK GM Science Review panel (July 2003).GM Science Review First Report 2013년 10월 16일 영국 GM Science Review 패널이 작성한 웨이백 머신에서 보관된 첫 보고서(2003년 7월) Chairman Professor Sir David King, Chief Scientific Advisor to the UK Government, P 9 데이비드 킹 교수, 영국 정부의 수석 과학 고문, P 9
- ^^ James C (1996). "Global Review of the Field Testing and Commercialization of Transgenic Plants: 1986 to 1995" (PDF). The International Service for the Acquisition of Agri-biotech Applications. Retrieved July 17, 2010.
- ^^ "Consumer Q&A". Fda.gov. March 6, 2009. Retrieved December 29, 2012.
- ^^ "AquAdvantage Salmon". FDA. Retrieved July 20, 2018.
- ^^ Nicolia, Alessandro; Manzo, Alberto; Veronesi, Fabio; Rosellini, Daniele (2013). "An overview of the last 10 years of genetically engineered crop safety research" (PDF). Critical Reviews in Biotechnology. 34 (1): 77–88. doi:10.3109/07388551.2013.823595. PMID 24041244. S2CID 9836802. We have reviewed the scientific literature on GE crop safety for the last 10 years that catches the scientific consensus matured since GE plants became widely cultivated worldwide, and we can conclude that the scientific research conducted so far has not detected any significant hazard directly connected with the use of GM crops.
The literature about Biodiversity and the GE food/feed consumption has sometimes resulted in animated debate regarding the suitability of the experimental designs, the choice of the statistical methods or the public accessibility of data. Such debate, even if positive and part of the natural process of review by the scientific community, has frequently been distorted by the media and often used politically and inappropriately in anti-GE crops campaigns. - ^^ "State of Food and Agriculture 2003–2004. Agricultural Biotechnology: Meeting the Needs of the Poor. Health and environmental impacts of transgenic crops". Food and Agriculture Organization of the United Nations. Retrieved August 30, 2019. Currently available transgenic crops and foods derived from them have been judged safe to eat and the methods used to test their safety have been deemed appropriate. These conclusions represent the consensus of the scientific evidence surveyed by the ICSU (2003) and they are consistent with the views of the World Health Organization (WHO, 2002). These foods have been assessed for increased risks to human health by several national regulatory authorities (inter alia, Argentina, Brazil, Canada, China, the United Kingdom and the United States) using their national food safety procedures (ICSU). To date no verifiable untoward toxic or nutritionally deleterious effects resulting from the consumption of foods derived from genetically modified crops have been discovered anywhere in the world (GM Science Review Panel). Many millions of people have consumed foods derived from GM plants – mainly maize, soybean and oilseed rape – without any observed adverse effects (ICSU).
- ^^ Ronald, Pamela (May 1, 2011). "Plant Genetics, Sustainable Agriculture and Global Food Security". Genetics. 188 (1): 11–20. doi:10.1534/genetics.111.128553. PMC 3120150. PMID 21546547. There is broad scientific consensus that genetically engineered crops currently on the market are safe to eat. After 14 years of cultivation and a cumulative total of 2 billion acres planted, no adverse health or environmental effects have resulted from commercialization of genetically engineered crops (Board on Agriculture and Natural Resources, Committee on Environmental Impacts Associated with Commercialization of Transgenic Plants, National Research Council and Division on Earth and Life Studies 2002). Both the U.S. National Research Council and the Joint Research Centre (the European Union's scientific and technical research laboratory and an integral part of the European Commission) have concluded that there is a comprehensive body of knowledge that adequately addresses the food safety issue of genetically engineered crops (Committee on Identifying and Assessing Unintended Effects of Genetically Engineered Foods on Human Health and National Research Council 2004; European Commission Joint Research Centre 2008). These and other recent reports conclude that the processes of genetic engineering and conventional breeding are no different in terms of unintended consequences to human health and the environment (European Commission Directorate-General for Research and Innovation 2010).
- ^^
But see also:그러나 다음 항목을 참조하십시오.
Domingo, José L.; Bordonaba, Jordi Giné (2011). "A literature review on the safety assessment of genetically modified plants" (PDF). Environment International. 37 (4): 734–742. doi:10.1016/j.envint.2011.01.003. PMID 21296423. In spite of this, the number of studies specifically focused on safety assessment of GM plants is still limited. However, it is important to remark that for the first time, a certain equilibrium in the number of research groups suggesting, on the basis of their studies, that a number of varieties of GM products (mainly maize and soybeans) are as safe and nutritious as the respective conventional non-GM plant, and those raising still serious concerns, was observed. Moreover, it is worth mentioning that most of the studies demonstrating that GM foods are as nutritional and safe as those obtained by conventional breeding, have been performed by biotechnology companies or associates, which are also responsible of commercializing these GM plants. Anyhow, this represents a notable advance in comparison with the lack of studies published in recent years in scientific journals by those companies.
Krimsky, Sheldon (2015). "An Illusory Consensus behind GMO Health Assessment". Science, Technology, & Human Values. 40 (6): 883–914. doi:10.1177/0162243915598381. S2CID 40855100. I began this article with the testimonials from respected scientists that there is literally no scientific controversy over the health effects of GMOs. My investigation into the scientific literature tells another story.
And contrast:그리고 대비:
Panchin, Alexander Y.; Tuzhikov, Alexander I. (January 14, 2016). "Published GMO studies find no evidence of harm when corrected for multiple comparisons". Critical Reviews in Biotechnology. 37 (2): 213–217. doi:10.3109/07388551.2015.1130684. ISSN 0738-8551. PMID 26767435. S2CID 11786594. Here, we show that a number of articles some of which have strongly and negatively influenced the public opinion on GM crops and even provoked political actions, such as GMO embargo, share common flaws in the statistical evaluation of the data. Having accounted for these flaws, we conclude that the data presented in these articles does not provide any substantial evidence of GMO harm.
The presented articles suggesting possible harm of GMOs received high public attention. However, despite their claims, they actually weaken the evidence for the harm and lack of substantial equivalency of studied GMOs. We emphasize that with over 1783 published articles on GMOs over the last 10 years it is expected that some of them should have reported undesired differences between GMOs and conventional crops even if no such differences exist in reality.and그리고
Yang, Y.T.; Chen, B. (2016). "Governing GMOs in the USA: science, law and public health". Journal of the Science of Food and Agriculture. 96 (4): 1851–1855. doi:10.1002/jsfa.7523. PMID 26536836. It is therefore not surprising that efforts to require labeling and to ban GMOs have been a growing political issue in the USA (citing Domingo and Bordonaba, 2011). Overall, a broad scientific consensus holds that currently marketed GM food poses no greater risk than conventional food... Major national and international science and medical associations have stated that no adverse human health effects related to GMO food have been reported or substantiated in peer-reviewed literature to date.
Despite various concerns, today, the American Association for the Advancement of Science, the World Health Organization, and many independent international science organizations agree that GMOs are just as safe as other foods. Compared with conventional breeding techniques, genetic engineering is far more precise and, in most cases, less likely to create an unexpected outcome. - ^^ "Statement by the AAAS Board of Directors On Labeling of Genetically Modified Foods" (PDF). American Association for the Advancement of Science. October 20, 2012. Retrieved August 30, 2019. The EU, for example, has invested more than €300 million in research on the biosafety of GMOs. Its recent report states: "The main conclusion to be drawn from the efforts of more than 130 research projects, covering a period of more than 25 years of research and involving more than 500 independent research groups, is that biotechnology, and in particular GMOs, are not per se more risky than e.g. conventional plant breeding technologies." The World Health Organization, the American Medical Association, the U.S. National Academy of Sciences, the British Royal Society, and every other respected organization that has examined the evidence has come to the same conclusion: consuming foods containing ingredients derived from GM crops is no riskier than consuming the same foods containing ingredients from crop plants modified by conventional plant improvement techniques.
Pinholster, Ginger (October 25, 2012). "AAAS Board of Directors: Legally Mandating GM Food Labels Could "Mislead and Falsely Alarm Consumers"" (PDF). American Association for the Advancement of Science. Retrieved August 30, 2019. - ^^ A decade of EU-funded GMO research (2001–2010) (PDF). Directorate-General for Research and Innovation. Biotechnologies, Agriculture, Food. European Commission, European Union. 2010. doi:10.2777/97784. ISBN 978-92-79-16344-9. Retrieved August 30, 2019.
- ^^ "AMA Report on Genetically Modified Crops and Foods (online summary)". American Medical Association. January 2001. Retrieved August 30, 2019. A report issued by the scientific council of the American Medical Association (AMA) says that no long-term health effects have been detected from the use of transgenic crops and genetically modified foods, and that these foods are substantially equivalent to their conventional counterparts. (from online summary prepared by ISAAA)" "Crops and foods produced using recombinant DNA techniques have been available for fewer than 10 years and no long-term effects have been detected to date. These foods are substantially equivalent to their conventional counterparts.
(from original report by AMA: [1])"REPORT 2 OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH (A-12): Labeling of Bioengineered Foods" (PDF). American Medical Association. 2012. Archived from the original (PDF) on September 7, 2012. Retrieved August 30, 2019. Bioengineered foods have been consumed for close to 20 years, and during that time, no overt consequences on human health have been reported and/or substantiated in the peer-reviewed literature. - ^^ "Restrictions on Genetically Modified Organisms: United States. Public and Scholarly Opinion". Library of Congress. June 30, 2015. Retrieved August 30, 2019. Several scientific organizations in the US have issued studies or statements regarding the safety of GMOs indicating that there is no evidence that GMOs present unique safety risks compared to conventionally bred products. These include the National Research Council, the American Association for the Advancement of Science, and the American Medical Association. Groups in the US opposed to GMOs include some environmental organizations, organic farming organizations, and consumer organizations. A substantial number of legal academics have criticized the US's approach to regulating GMOs.
- ^^ National Academies Of Sciences, Engineering; Division on Earth Life Studies; Board on Agriculture Natural Resources; Committee on Genetically Engineered Crops: Past Experience Future Prospects (2016). Genetically Engineered Crops: Experiences and Prospects. The National Academies of Sciences, Engineering, and Medicine (US). p. 149. doi:10.17226/23395. ISBN 978-0-309-43738-7. PMID 28230933. Retrieved August 30, 2019. Overall finding on purported adverse effects on human health of foods derived from GE crops: On the basis of detailed examination of comparisons of currently commercialized GE with non-GE foods in compositional analysis, acute and chronic animal toxicity tests, long-term data on health of livestock fed GE foods, and human epidemiological data, the committee found no differences that implicate a higher risk to human health from GE foods than from their non-GE counterparts.
- ^^ "Frequently asked questions on genetically modified foods". World Health Organization. Retrieved August 30, 2019. Different GM organisms include different genes inserted in different ways. This means that individual GM foods and their safety should be assessed on a case-by-case basis and that it is not possible to make general statements on the safety of all GM foods.
GM foods currently available on the international market have passed safety assessments and are not likely to present risks for human health. In addition, no effects on human health have been shown as a result of the consumption of such foods by the general population in the countries where they have been approved. Continuous application of safety assessments based on the Codex Alimentarius principles and, where appropriate, adequate post market monitoring, should form the basis for ensuring the safety of GM foods. - ^^ Haslberger, Alexander G. (2003). "Codex guidelines for GM foods include the analysis of unintended effects". Nature Biotechnology. 21 (7): 739–741. doi:10.1038/nbt0703-739. PMID 12833088. S2CID 2533628. These principles dictate a case-by-case premarket assessment that includes an evaluation of both direct and unintended effects.
- ^^ Some medical organizations, including the British Medical Association, advocate further caution based upon the precautionary principle:영국 의료협회를 포함한 일부 의료단체는 예방원칙에 근거한 추가적인 주의를 주장한다.
"Genetically modified foods and health: a second interim statement" (PDF). British Medical Association. March 2004. Retrieved August 30, 2019. In our view, the potential for GM foods to cause harmful health effects is very small and many of the concerns expressed apply with equal vigour to conventionally derived foods. However, safety concerns cannot, as yet, be dismissed completely on the basis of information currently available.
When seeking to optimise the balance between benefits and risks, it is prudent to err on the side of caution and, above all, learn from accumulating knowledge and experience. Any new technology such as genetic modification must be examined for possible benefits and risks to human health and the environment. As with all novel foods, safety assessments in relation to GM foods must be made on a case-by-case basis.
Members of the GM jury project were briefed on various aspects of genetic modification by a diverse group of acknowledged experts in the relevant subjects. The GM jury reached the conclusion that the sale of GM foods currently available should be halted and the moratorium on commercial growth of GM crops should be continued. These conclusions were based on the precautionary principle and lack of evidence of any benefit. The Jury expressed concern over the impact of GM crops on farming, the environment, food safety and other potential health effects.
The Royal Society review (2002) concluded that the risks to human health associated with the use of specific viral DNA sequences in GM plants are negligible, and while calling for caution in the introduction of potential allergens into food crops, stressed the absence of evidence that commercially available GM foods cause clinical allergic manifestations. The BMA shares the view that there is no robust evidence to prove that GM foods are unsafe but we endorse the call for further research and surveillance to provide convincing evidence of safety and benefit. - ^^ Funk, Cary; Rainie, Lee (January 29, 2015). "Public and Scientists' Views on Science and Society". Pew Research Center. Retrieved August 30, 2019. The largest differences between the public and the AAAS scientists are found in beliefs about the safety of eating genetically modified (GM) foods. Nearly nine-in-ten (88%) scientists say it is generally safe to eat GM foods compared with 37% of the general public, a difference of 51 percentage points.
- ^^ Marris, Claire (2001). "Public views on GMOs: deconstructing the myths". EMBO Reports. 2 (7): 545–548. doi:10.1093/embo-reports/kve142. PMC 1083956. PMID 11463731.
- ^^ Final Report of the PABE research project (December 2001). "Public Perceptions of Agricultural Biotechnologies in Europe". Commission of European Communities. Archived from the original on May 25, 2017. Retrieved August 30, 2019.
- ^^ Scott, Sydney E.; Inbar, Yoel; Rozin, Paul (2016). "Evidence for Absolute Moral Opposition to Genetically Modified Food in the United States" (PDF). Perspectives on Psychological Science. 11 (3): 315–324. doi:10.1177/1745691615621275. PMID 27217243. S2CID 261060.
- ^^ "Restrictions on Genetically Modified Organisms". Library of Congress. June 9, 2015. Retrieved August 30, 2019.
- ^^ Bashshur, Ramona (February 2013). "FDA and Regulation of GMOs". American Bar Association. Archived from the original on June 21, 2018. Retrieved August 30, 2019.
- ^^ Sifferlin, Alexandra (October 3, 2015). "Over Half of E.U. Countries Are Opting Out of GMOs". Time. Retrieved August 30, 2019.
- ^^ Lynch, Diahanna; Vogel, David (April 5, 2001). "The Regulation of GMOs in Europe and the United States: A Case-Study of Contemporary European Regulatory Politics". Council on Foreign Relations. Retrieved August 30, 2019.
- ^^ Pollack A (April 13, 2010). "Study Says Overuse Threatens Gains From Modified Crops". New York Times.
- ^^ Industrial Biotechnology and Biomass Utilisation Archived April 5, 2013, at the Wayback Machine2013년 4월 5일 웨이백머신에 보관된 산업 생명공학 및 바이오매스 활용
- ^^ "Industrial biotechnology, A powerful, innovative technology to mitigate climate change". Archived from the original on January 2, 2014. Retrieved January 1, 2014.
- ^^ Daniel A.다니엘 A. Vallero, Environmental Biotechnology: Vallero, 환경 생명공학: A Biosystems Approach, Academic Press, Amsterdam, NV; ISBN 978-0-12-375089-1; 2010. A Biosystems 접근방식, NV, 암스테르담, 아카데미 프레스; ISBN 978-0-12-375089-1; 2010.
- ^^ Gaskell G, Bauer MW, Durant J, Allum NC (July 1999). "Worlds apart? The reception of genetically modified foods in Europe and the U.S". Science. 285 (5426): 384–7. doi:10.1126/science.285.5426.384. PMID 10411496. S2CID 5131870.
- ^^ "The History and Future of GM Potatoes". Potato Pro. March 10, 2010.
- ^^ Wesseler J, Kalaitzandonakes N (2011). "Present and Future EU GMO policy". In Oskam A, Meesters G, Silvis H (eds.). EU Policy for Agriculture, Food and Rural Areas (2nd ed.). Wageningen: Wageningen Academic Publishers. pp. 23–332.
- ^^ Beckmann VC, Soregaroli J, Wesseler J (2011). "Coexistence of genetically modified (GM) and non-modified (non GM) crops: Are the two main property rights regimes equivalent with respect to the coexistence value?". In Carter C, Moschini G, Sheldon I (eds.). Genetically modified food and global welfare. Frontiers of Economics and Globalization Series. 10. Bingley, UK: Emerald Group Publishing. pp. 201–224.
- ^^ "Biotechnology Predoctoral Training Program". National Institute of General Medical Sciences. December 18, 2013. Retrieved October 28, 2014.
External links외부 링크[[edit편집]]
| Wikimedia Commons has media related to Biotechnology.위키미디어 커먼즈에는 생명공학 관련 미디어가 있다. |
| At Wikiversity, you can learn more and teach others about Biotechnology at the Department of Biotechnology위키다양성에서는 생명공학과에서 생명공학과에 대해 더 많이 배우고 다른 사람들에게 가르칠 수 있다. |
- Foundation for Biotechnology Awareness and Education,생명공학 인식과 교육을 위한 재단
- A report on Agricultural Biotechnology focusing on the impacts of "Green" Biotechnology with a special emphasis on economic aspects. fao.org.농업생명공학에 관한 보고서는 "녹색"생명공학이 경제적 측면에 특별히 중점을 두고 있다. fao.org
- US Economic Benefits of Biotechnology to Business and Society NOAA Economics, economics.noaa.gov생명공학이 비즈니스 및 사회 NOAA 경제학에 미치는 미국의 경제적 이점, economics.noaa.gov
- Database of the Safety and Benefits of Biotechnology – a database of peer-reviewed scientific papers and the safety and benefits of biotechnology.생명공학의 안전과 이익 데이터베이스 – 동료 검토 과학 논문과 생명공학의 안전과 이익의 데이터베이스.
- What is Biotechnology?생명공학이란 무엇인가? – A curated collection of resources about the people, places and technologies that have enabled biotechnology to transform the world we live in today – 생명공학이 오늘날 우리가 살고 있는 세상을 변화시킬 수 있도록 한 인력, 장소 및 기술에 대한 자원의 큐레이션된 모음
hide .mw-parser-output .navbar{display:inline;font-size:88%;font-weight:normal}.mw-parser-output .navbar-collapse{float:left;text-align:left}.mw-parser-output .navbar-boxtext{word-spacing:0}.mw-parser-output .navbar ul{display:inline-block;white-space:nowrap;line-height:inherit}.mw-parser-output .navbar-brackets::before{margin-right:-0.125em;content:"[ "}.mw-parser-output .navbar-brackets::after{margin-left:-0.125em;content:" ]"}.mw-parser-output .navbar li{word-spacing:-0.125em}.mw-parser-output .navbar-mini abbr{font-variant:small-caps;border-bottom:none;text-decoration:none;cursor:inherit}.mw-parser-output .navbar-ct-full{font-size:114%;margin:0 7em}.mw-parser-output .navbar-ct-mini{font-size:114%;margin:0 4em}.mw-parser-output .infobox .navbar{font-size:100%}.mw-parser-output .navbox .navbar{display:block;font-size:100%}.mw-parser-output .navbox-title .navbar{float:left;text-align:left;margin-right:0.5em}
Biotechnology생명공학History역사Branches나뭇가지Biological concepts생물학적 개념General concepts일반개념Basic techniques기본기법and tools 도구 및 도구Applications적용들Interdisciplinary 학제간 fields분야ListsLists
Topics related to biology생물학과 관련된 주제People and history사람과 역사Institutions, publications기관, 출판물Terms and phrases용어 및 구Related disciplines관련 분야Other다른
|
Authority control권한통제
![]()
<img src="//en.wikipedia.org/wiki/Special:CentralAutoLogin/start?type=1x1" alt="" title="" width="1" height="1" style="border: none; position: absolute;">
Retrieved from "https://en.wikipedia.org/w/index.php?title=Biotechnology&oldid=1006750217""https://en.wikipedia.org/w/index.php?title=Biotechnology&oldid=1006750217"에서 검색됨
Hidden categories: 숨겨진 카테고리:
- Webarchive template wayback linksWebarchive 템플릿 웨이백
- CS1 maint: archived copy as titleCS1 유지 관리: 제목으로 보관된 복사본
- CS1: long volume valueCS1: 긴 볼륨 값
- Articles with short description설명이 짧은 기사
- Short description matches Wikidata간단한 설명이 위키다타와 일치함
- Use mdy dates from September 20182018년 9월부터 mdy 날짜 사용
- Commons category link from WikidataWikidata의 공용 카테고리 링크
- Wikipedia articles with GND identifiersGND 식별자가 있는 위키백과 기사
- Wikipedia articles with HDS identifiersHDS 식별자가 있는 위키백과 기사
- Wikipedia articles with LCCN identifiersLCCN 식별자가 있는 위키백과 기사
- Wikipedia articles with NDL identifiersNDL 식별자가 있는 위키백과 문서
Navigation menu내비게이션 메뉴
Personal tools개인 도구
- Not logged in로그인되지 않음
- TalkTalk
- Contributions기부금
- Create account계정 만들기
- Log in로그 인.
Namespaces네임스페이스
VariantsVariants
Views보기
More많은
Search검색
Navigation항법
- Main page메인 페이지
- Contents내용물
- Current events현재 이벤트
- Random article임의품
- About Wikipedia위키백과 정보
- Contact us우리에게 연락하세요
- Donate기부하다.
Contribute기부하다
Tools도구들
- What links here여기에 링크된 내용
- Related changes관련변경
- Upload file파일 업로드
- Special pages특수 페이지
- Permanent link영구 링크
- Page information페이지 정보
- Cite this page이 페이지를 인용하십시오.
- Wikidata item위키다타 아이템
Print/export인쇄/수출
In other projects다른 프로젝트에서
Languages언어들
- Afrikaans아프리칸 스어
- Alemannisch알레마니슈
- العربيةالعربية
- Aragonés아라고네스
- অসমীয়াঅসমীয়া
- AsturianuAsturianu
- Azərbaycanca아즈르바이칸카
- تۆرکجهتۆرکجه
- বাংলাবাংলা
- Bân-lâm-gú반롬구
- БашҡортсаБашҡортса
- БеларускаяБеларуская
- Беларуская (тарашкевіца)Беларуская (тарашкевіца)
- БългарскиБългарски
- BoarischBoarisch
- BosanskiBosanski
- CatalàCatalà
- ČeštinaČeština
- CymraegCymraeg
- Dansk덴마크
- Deutsch도이치
- EestiEesti
- ΕλληνικάΕλληνικά
- Español력 산업 회의
- Esperanto에스페란토
- EuskaraEuskara
- فارسیفارسی
- Français프랑세
- GaeilgeGaeilge
- GalegoGalego
- 贛語贛語
- ગુજરાતીગુજરાતી
- 한국어한국어
- ՀայերենՀայերեն
- हिन्दीहिन्दी
- HrvatskiHrvatski
- Ido그렇습니다.
- IlokanoIlokano
- Bahasa Indonesia인도네시아 바하사
- Interlingua인터링구아
- ÍslenskaÍslenska
- Italiano이탈리아노
- עבריתעברית
- Jawa자와
- ಕನ್ನಡಕನ್ನಡ
- ქართულიქართული
- ҚазақшаҚазақша
- Kiswahili스와힐리 말
- Kriyòl gwiyannen크리힐 귀야넨
- KurdîKurdî
- КыргызчаКыргызча
- Latina라틴계 여자
- LatviešuLatviešu
- LietuviųLietuvių
- LimburgsLimburgs
- Livvinkarjala리브빈카르얄라
- Magyar마자르 말
- МакедонскиМакедонски
- മലയാളംമലയാളം
- मराठीमराठी
- Bahasa Melayu바하사멜라유
- МонголМонгол
- မြန်မာဘာသာမြန်မာဘာသာ
- Nederlands네덜란드
- नेपाल भाषानेपाल भाषा
- 日本語日本語
- НохчийнНохчийн
- Norsk bokmål노르스크 보크멜
- Norsk nynorsk노르스크 니노르스크
- Occitan오크어
- Oʻzbekcha/ўзбекчаOʻzbekcha/ўзбекча
- ਪੰਜਾਬੀਪੰਜਾਬੀ
- پنجابیپنجابی
- Patois크리올
- PolskiPolski
- PortuguêsPortuguês
- RomânăRomână
- РусиньскыйРусиньскый
- РусскийРусский
- Саха тылаСаха тыла
- ShqipShqip
- සිංහලසිංහල
- Simple English심플 잉글리쉬
- Slovenčina슬로베니치나
- Slovenščina슬로베니슈치나
- کوردیکوردی
- Српски / srpskiсрп и / srpski
- Srpskohrvatski / српскохрватскиSrpskohrvatski / срп ооаорааааа
- Sunda순다
- Suomi수오미. Finland의 핀란드 이름.
- SvenskaSvenska
- Tagalog따갈로그
- தமிழ்தமிழ்
- Татарча/tatarçaт바사샤르크레스카/타타르사
- తెలుగుతెలుగు
- ไทยไทย
- ТоҷикӣТоҷикӣ
- TürkçeTürkçe
- УкраїнськаУкраїнська
- اردواردو
- Tiếng Việt톈궁 비엣
- WinarayWinaray
- 吴语吴语
- ייִדישייִדיש
- 粵語粵語
- Žemaitėška제마이트슈카
- 中文中文 98 more
'생명공학' 카테고리의 다른 글
| lower respiratory tract (0) | 2021.09.02 |
|---|---|
| upper respiratory tract (0) | 2021.09.02 |
| life - What is Life (0) | 2021.05.01 |
| Molecular Biology (0) | 2021.03.31 |
| 분자생물학 (0) | 2021.03.07 |




